Le Châtelier's Principle
Overview
Source: Laboratory of Dr. Lynne O'Connell — Boston College
When the conditions of a system at equilibrium are altered, the system responds in such a way as to maintain the equilibrium. In 1888, Henri-Lewis Le Châtelier described this phenomenon in a principle that states, "When a change in temperature, pressure, or concentration disturbs a system in chemical equilibrium, the change will be counteracted by an alteration in the equilibrium composition."
This experiment demonstrates Le Châtelier's principle at work in a reversible reaction between iron(III) ion and thiocyanate ion, which produces iron(III) thiocyante ion:
Fe3+(aq) + SCN- (aq)  FeSCN2+ (aq)
FeSCN2+ (aq)
The concentration of one of the ions is altered either by directly adding a quantity of one ion to the solution or by selectively removing an ion from the solution through formation of an insoluble salt. Observations of color changes indicate whether the equilibrium has shifted to favor formation of the products or the reactants. In addition, the effect of a temperature change on the solution at equilibrium can be observed, which leads to the ability to conclude whether the reaction is exothermic or endothermic.
Principles
To fully understand Le Châtelier's Principle, a reversible reaction of the sort expressed by the following chemical equation is considered:
aA + bB  cC + dD
cC + dD
This reaction actually consists of two competing processes: the forward reaction, in which the products C and D are formed from the reactants, and the reverse reaction, in which the reactants A and B are formed from the products. When the rates of these two processes equal each other, there is no net change in the concentration of either the products or the reactants, and the reaction is said to be at equilibrium. The ratio of the equilibrium concentrations of the products to the equilibrium concentrations of the reactants is a constant, as shown by the following equation:

where Kc is the equilibrium constant. The brackets signify the concentrations of the various species, and the lowercase letters represent the number of moles of each substance involved in the balanced equation. In the case of the reaction between iron(III) and thiocyanate ions shown previously, the equilibrium constant is:
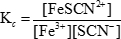
When the concentration of either a reactant or a product in an equilibrium solution is altered, the concentrations of the other species must change in order to maintain the constant ratio of products to reactants. These changes are referred to as "shifts" in the equilibrium. The equilibrium can either shift to the left, meaning it proceeds in the reverse direction and the concentrations of the reactants increase, or shift to the right, meaning it proceeds in the forward direction and the concentrations of the products increase. In the reaction between iron(III) and thiocyanate ions, a shift to the left would mean formation of more iron(III) and thiocyanate ions, while a shift to the right would mean formation of more iron(III) thiocyanate ions.
The equilibrium constant is dependent upon the temperature; thus, a change in the temperature of an equilibrium solution can also result in a shift to the right or left, depending on whether the reaction is exothermic or endothermic. For an exothermic reaction, the heat generated by the reaction can be represented as residing on the product side of the equation, since heat is produced along with the products:
aA + bB  cC + dD + heat
cC + dD + heat
If heat is added to the system by increasing the temperature, the equilibrium shifts to the left, and the concentrations of the reactants increase. For an endothermic reaction, the addition of heat would result in a shift to the right.
aA + bB + heat  cC + dD
cC + dD
In this case, the concentrations of the reactants would increase with an increase in temperature.
Procedure
1. Preparation of the Iron(III) Thiocyanate Equilibrium Solutions
- Place 1 drop of 1 M Fe(NO3)3 solution in a test tube and dilute with 2 mL of water. Place 1 drop of 1 M KSCN in another test tube and dilute with 2 mL of water. These two test tubes serve as controls to compare against the other test tubes.
- Place 1 drop of 1 M Fe(NO3)3 solution in a test tube.
- Add 1 drop of 1 M KSCN to the test tube.
- Add 16 mL of water to the test tube and thoroughly mix the contents.
- Record any observations.
- Divide the mixture into 2 mL portions in 8 test tubes. One of the test tubes remains untouched and serves as a FeSCN2+ control. Number the other test tubes 1–7.
2. Addition of Iron(III) and Thiocyanate Ions to the Equilibrium Solution
- To test tube 1, add 1 drop of 1 M Fe(NO3)3 solution.
- Shake to mix and record any observations.
- To test tube 2, add 1 drop of 1 KSCN solution.
- Shake to mix and record any observations.
3. Addition of Silver Nitrate to the Equilibrium Solution
- To test tube 3, add 3 drops of 0.1 M AgNO3 solution.
- Shake to mix and record any observations.
- Add 3 drops of 1 M Fe(NO3)3 to the test tube.
- Shake to mix and record any observations.
- To test tube 4, add 3 drops of 0.1 M AgNO3 solution.
- Shake to mix and record any observations.
- Add 3 drops of 1 M KSCN to the test tube.
- Shake to mix and record any observations.
4. Addition of Potassium Phosphate to the Equilibrium Solution
- To test tube 5, add 3 drops of 0.5 M K3PO4 solution.
- Shake to mix and record any observations.
- Add 3 drops of 1 M Fe(NO3)3 to the test tube.
- Shake to mix and record any observations.
- To test tube 6, add 3 drops of 0.5 M K3PO4 solution.
- Shake to mix and record any observations.
- Add 3 drops of 1 M KSCN to the test tube.
- Shake to mix and record any observations.
5. Changing the Temperature of the Equilibrium Solution
- Place test tube 7 in a 70–80 °C water bath for 1–2 min.
- Compare the warm solution to the solution in the unheated test tube (the FeSCN2+ control), and record any observations.
- Collect the contents of test tubes 3 and 4 in the laboratory waste jar labeled "Silver." Pour the contents of all the other test tubes down the drain.
Results
Observations of the initial solutions and the mixture of the two solutions can be seen in Table 1.
Observations of the equilibrium mixtures upon addition of various reagents can be seen in Table 2.
Observation when the temperature is changed: In test tube 7, the solution turns more orange in color (less red, more yellow) when heated.
In test tubes 1 and 2, when iron(III) nitrate, which contains a reactant, was added to the equilibrium solution, the red color of the solution intensified. This observation indicates that the equilibrium shifted to the right as concentration of the product, iron(III) thiocyanate ion, increased. Similarly, when potassium thiocyanate, which contains the other reactant, was added to the equilibrium solution, the red color of the solution intensified. This observation also indicates that the equilibrium shifted to the right as concentration of the product increased.
In test tubes 3 and 4, when silver nitrate (AgNO3) was added to the equilibrium solution, the red color of the product faded and the solution became colorless. This observation indicates that the equilibrium shifted to the left as the concentration of reactants increased. In addition, a precipitate was observed. The red color reappeared upon addition of thiocyanate ion (SCN-). This observation indicates that the equilibrium shifted to the right as the concentration of the product increased. The red color did not reappear when iron(III) ion (Fe3+) was added.
From these observations, it can be concluded that silver thiocyanate (AgSCN) was the precipitate that formed when silver nitrate was added to the equilibrium solution. The formation of this solid is responsible for the cloudiness observed in both test tubes. When the thiocyanate ion was removed from the solution by precipitation, the equilibrium shifted to the left, because the concentration of one of the reactants had been reduced. When more thiocyanate ion was then added, the equilibrium shifted back to the right to re-establish the equilibrium ratio of concentrations by re-forming iron(III) thiocyanate. The addition of more iron(III) ion did not shift the equilibrium back to the right, because the thiocyanate ion had been removed from the solution as silver thiocyanate precipitate and was no longer available to react with iron(III) to form the iron(III) thiocyanate ion.
In test tubes 5 and 6, when potassium phosphate ion (K3PO4) was added to the equilibrium solution, the red color of the products faded and the solution became yellow. This observation indicates that the equilibrium shifted to the left as the concentration of reactants increased. The red color reappeared upon addition of iron(III) ion (Fe3+). This observation indicates that the equilibrium shifted to the right as the concentration of the product increased. In addition, a precipitate was observed. The red color did not reappear when the thiocyanate ion (SCN-) was added.
From these observations, it can be concluded that iron(III) phosphate (FePO4) salt was formed when potassium phosphate was added to the equilibrium solution. When the iron(III) ion was removed from the solution by formation of this salt, the equilibrium shifted to the left, because the concentration of one of the reactants had been reduced. When more iron(III) ion was then added, the equilibrium shifted back to the right to re-establish the equilibrium ratio of concentrations by re-forming iron(III) thiocyante. Although no cloudiness was detected by eyesight when the phosphate ion was initially added, a cloudiness did appear when the iron(III) ion was subsequently added, which is the solid iron(III) phosphate salt. The addition of more thiocyanate ion did not shift the equilibrium back to the right, because the iron(III) ion had been removed from the solution as iron(III) phosphate salt and was no longer available to react with the thiocyanate ion to form the iron(III) thiocyanate ion.
In test tube 7, as the temperature increased, the red color of the products faded, indicating an equilibrium shift to the left as more reactants were formed. This observation leads to the conclusion that the reaction is exothermic. For an exothermic reaction, the heat generated by the reaction resides on the product side of the equation:
Fe3+ + SCN-  FeSCN2+ + heat
FeSCN2+ + heat
When heat was added to the system (by increasing the temperature), the equilibrium shifted to the left.
| Solution | Observation |
| Fe(NO3)3 | Yellow, clear |
| KSCN | Colorless, clear |
| Fe(SCN)2+ | Orange-red, clear |
Table 1. Observations of the initial solutions and the mixture of the two solutions.
| Test Tube # | First Reagent | Observation of Equilibrium Solution | Second Reagent | Observation of Equilibrium Solution |
| 1 | Fe(NO3)3 | Red, clear | — | — |
| 2 | KSCN | Red, clear | — | — |
| 3 | AgNO3 (colorless, clear) | Colorless (white), cloudy | Fe(NO3)3 | Yellow, still cloudy |
| 4 | AgNO3 | Colorless (white), cloudy | KSCN | Orange-red, still cloudy |
| 5 | K3PO4 (colorless, clear) | Yellow, clear | Fe(NO3)3 | Orange-red, cloudy |
| 6 | K3PO4 | Yellow, clear | KSCN | Yellow, still clear |
Table 2. Observations of the equilibrium mixtures upon addition of various reagents.
Application and Summary
Le Châtelier's principle is at work in human bodies. Oxygen is transported from the lungs to muscle and other tissues by a protein called hemoglobin (Hb) that is found in the blood. The oxygen molecule binds to this protein in a reversible reaction that can be described by an equilibrium equation:
Hb + 4 O2  Hb(O2)4
Hb(O2)4
In the lungs, the partial pressure of oxygen gas is high (on the order of 100 torr). The equilibrium shifts to the right in this environment, and the oxygen molecules bind to hemoglobin molecules until the protein is saturated with oxygen. When this saturated hemoglobin reaches the cells of muscle tissue, where the pressure of oxygen is much lower, the equilibrium shifts to the left, and the oxygen is released. If the muscle is at rest, the oxygen pressure is about 30 torr, and approximately 40% of the oxygen is released. When the muscle is active, the oxygen pressure ranges from 3 to 18 torr, and about 85% of the oxygen is released to satisfy the increased metabolic demand.
Another physiological example of an equilibrium system involves the regulation of blood pH. Carbon dioxide in the blood reacts reversibly with water to produce carbonic acid, which dissociates to produce hydronium and bicarbonate ions:
CO2 (aq) + H2O (l)  H2CO3 (aq)
H2CO3 (aq)  H3O+ (aq) + HCO3-(aq)
H3O+ (aq) + HCO3-(aq)
During strenuous exercise, the amount of carbon dioxide produced by the cells increases as a result of high metabolic activity. The increased concentration of carbon dioxide in the blood causes a shift to the right in this equilibrium to produce more carbonic acid. When this happens, the pH level of the blood decreases as hydronium ion concentration increases. One of the body's responses to this imbalance in blood pH is to increase the rate of breathing so more carbon dioxide gas is exhaled from the lungs, thus shifting the equilibrium back to the left and raising the pH back to normal levels.
Le Châtelier's principle must also be taken into account in many industrial processes. Ammonia is an important chemical used in fertilizers, cleaning agents, and as a building block in synthetic organic reactions. The industrial production of ammonia is accomplished using the Haber process, which relies on the reversible reaction between hydrogen and nitrogen:
3 H2 (g) + N2 (g)  2 NH3 (g)
2 NH3 (g)
In order to optimize the production of ammonia, the reaction is run at high pressure, usually around 200 atm. There are 4 moles of gas on the left-hand side of the equation and 2 moles of gas on the right-hand side. Le Châtelier's principle dictates that an increase of pressure on the system shifts the equilibrium to the right, because the volume of 2 moles of gas is smaller than the volume of 4 moles of gas. Since volume and pressure are directly proportional, a shift to reduce volume also reduces pressure, and the system returns to equilibrium. In addition, the process involves liquefying the ammonia gas in a condenser, so it is removed from the reaction chamber. This decrease in ammonia also shifts the equilibrium to the right, maximizing the amount of ammonia produced.
Skip to...
Videos from this collection:

Now Playing
Le Châtelier's Principle
General Chemistry
265.8K Views

Common Lab Glassware and Uses
General Chemistry
659.0K Views

Solutions and Concentrations
General Chemistry
275.4K Views

Determining the Density of a Solid and Liquid
General Chemistry
556.9K Views

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
383.8K Views

Determining the Empirical Formula
General Chemistry
183.8K Views

Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.6K Views

Using a pH Meter
General Chemistry
346.9K Views

Introduction to Titration
General Chemistry
425.7K Views

Ideal Gas Law
General Chemistry
79.4K Views

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.8K Views

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.8K Views

Determining Rate Laws and the Order of Reaction
General Chemistry
196.4K Views

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.8K Views

Coordination Chemistry Complexes
General Chemistry
91.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved