需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Monitoring the Brain Cortex with a Multi-Electrode Array in Response to Seizure-Inducing Agents
Overview
In this video, the effects of transcranial direct-current stimulation (tDCS) on brain slices containing the anterior cingulate cortex (ACC) and thalamus regions are investigated, using a multi-electrode array (MEA) to measure electric signals. By delivering electric pulses and infusing seizure-inducing drugs, the study investigates the ACC response to seizures.
研究方案
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Preparing Experimental Solution and Equipment for MEA (Multielectrode Array) Recording
- Prepare artificial cerebral spinal fluid (aCSF; 124 mM NaCl, 4.4 mM KCl, 1 mM NaH2PO3, 2 mM MgSO4, 2 mM CaCl2, 25 mM NaHCO3, and 10 mM glucose, bubbled with 95% O2 and 5% CO2).
- Use two types of MEA probes: 6 x 10 planar MEA and 8 x 8 MEA. The former probe covers the region that comprises the cortex, striatum, and thalamus. The latter probe covers only the cortical region.
- Use a 60-channel amplifier with a band-pass filter set between 0.1 Hz and 3 kHz at 1,200 amplification. Acquire data at a 10 kHz sampling rate.
- Place two AgCl-coated silver wires inside the MEA chamber for DCS( Direct current stimulation). Then, use the AgCl-coated silver wires to produce electric fields generated by an isolated stimulator.
- Place a tungsten electrode (diameter, 127 µm; length, 7.62 cm; 8° AC tapered tip; resistance, 5 MΩ) for thalamic stimulation, and place the reference electrode in the MEA chamber. Deliver the tungsten electrode's currents using an isolated stimulator that is controlled by a pulse generator (Figure 1).
2. Brain Slice Preparation
- Use male C57BL/6J mice, 4-8 weeks old. House the animals in an air-conditioned room (21-23 °C; 50% humidity; 12 hr/12 hr light /dark cycle, lights on at 8:00 AM) with free access to food and water.
- Take a 250 ml aliquot of the aCSF prepared in Step 1.1 and place it in an ice-filled beaker. At the same time, supply continuous gas composed of 95% O2 and 5% CO2.
- Surgery
- Anesthetize the animal with 4% isoflurane in a glass box for approximately 3 min. Once the animal reaches a surgical depth of anesthesia (indicated by the lack of a response to toe pinch), place it on a shallow tray that is filled with crushed ice and remove the head using scissors.
- Expose the skull and trim off the remaining muscle. Next, using rongeurs, peel away the dorsal surface of the skull from the brain. Trim away the sides of the skull using rongeurs. Sterilize all of the surgical instruments with a 75% ethanol solution.
- Using a spatula, cut the olfactory bulbs and nerve connections along the ventral surface of the brain and remove the brain. After decapitation, and quickly transfer the brain to a beaker filled with ice-cold oxygenated aCSF.
- Preparation of Medial Thalamus (MT)-ACC (Anterior cingulate cortex) Brain Slice
Note: Prepare slices that contain the pathway from the MT to ACC- Hand-cut the brain block with two sagittal cuts 2.0 mm lateral to the midline in each hemisphere to display the subcortical anatomy. Then, make two angled cuts. Make the first cross-cut parallel to the visible fiber tract in the striatum.
- Make the second cross-cut from the connection between the cerebellum and visual cortex to the midpoint between the anterior commissure and optic tract that are ventral and parallel to the thalamocingulate pathway.
- Attach the brain block to an angular plate (~120°) with cyanoacrylate adhesive and make a cut just above the turning point of the pathway. Unfold the plate, flatten it, and glue it onto the chamber stage of a vibratome.
- Make medial thalamus-ACC brain slices (500 µm thick) and then immerse them in ice-cold oxygenated aCSF.Transfer slices to the recording chamber and keep at 32 °C under continuous perfusion (12 ml/min) with oxygenated aCSF for 1 hr.
3. Preparation of Perfusion Chamber for Multielectrode Array Recording
- Preparation of Perfusion Chamber
- Place an MEA probe on a multi-channel system, and use two separate polyethylene tubes to connect the probe to a peristaltic pump. Use one tube to guide the aCSF into the MEA chamber and the other tube to guide the aCSF out of the chamber. Finally, continuously perfuse the preparation with warm (29-30 °C) oxygenated aCSF (8 ml/min).
- Transfer brain slice to MEA. Hold down the brain slice on the MEA using a wet cotton swab. Carefully move the brain slice to ensure the ACC is oriented above the electrodes.
- Use slice anchor kits and hold-downs to press the brain slice. This step ensures a good electrical connection between the slice and electrodes.
4. Generation of Electric Fields by DCS
Note: The definition of the electric field orientation was based on the direction of the axodendritic axis in the ACC. The orientations of dendrite and soma compartments were confirmed using Golgi staining12.
- Place the AgCl electrode (defined as the anode) proximal to the ACC and place the other electrode (defined as the cathode) distal to the ACC. Record the field strength that is generated by the two field orientations (parallel and perpendicular to the ACC axodendritic fibers) by the MEA, and deliver the currents of the electric fields using a stimulator.
- Fix the distance of the AgCl electrodes (about 1.5-2 cm), and adjust the stimulator's current strength to make the DCS between 0.5 and 2 mA.
5. Electrically-induced Cortical Synaptic Responses
Note: Induce synaptic responses in the ACC by electrical stimulation in the MT, in which a programmable electrical stimulus generator produces rectangular biphasic current pulses.
- Repeat Section 3 above.
- Place a tungsten electrode in the MT, and deliver pulses from the stimulator to the thalamic region of the slices via bipolar tungsten electrodes.
- Use various current intensities to determine the threshold that elicits an ACC response. Here, use an intensity of ±150 µA and duration of 200 µsec, which elicited an 80% maximal response in the ACC in most slices.
- Move the tungsten electrode along the thalamocingulate pathway (from MT to corpus callosum) in the MT-ACC slice to obtain the optimal response profiles.
- Make 10-20 sweeps of ACC responses, and use the software to automatically average all of the ACC evoked by MT stimulation. The result iss the synaptic responses in ACC induced from MT stimulation by MT-ACC pathway.
6. Electrically-induced Seizure-like Activity
Note: Seizure-like activity was induced by the application of 4-aminopyridine (4-AP; 250 µM) and bicuculline (5 µM). Previous time-control studies showed that maximal and stable responses appeared 2-3 hr after drug application14.
- Repeat Section 5 above.
- Add drugs to the perfusion solution. Use 4-AP (4-Aminopyridine)(250 µM) and bicuculline (5 µM). Mix the drugs uniformly and continue perfusion for 2-3 hr.
- To facilitate seizure-like activity, maintain the perfusion pump at a relatively fast perfusion rate (8 ml/min), which can also help prevent the build-up of a pH gradient.
- Place a tungsten electrode in the MT, and deliver electrical stimulation (150 µA, 200 µsec duration) to obtain ACC response profiles.
- Make 10-20 sweeps and average the responses.
- Replace the perfusion solution with fresh aCSF to wash out the drugs. Repeat Step 6.5.
结果
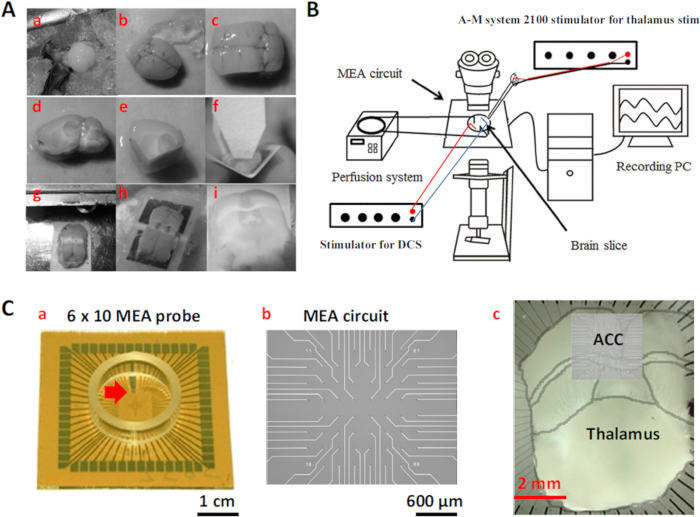
Figure 1: Preparation of the Thalamocingulate Slice and MEA Recording System Setup. (A) MT-ACC slice procedure (a) Remove the brain and (b) transfer to cool oxygenated aCSF. (c) Make two parasagittal cuts from the midline. (d) Side view of the medial part of the brain block. (e) Make two angled ventral cuts of the brain block. (f) Glue the brain block onto an angled plastic plate. (g) Make a dors...
披露声明
材料
| Name | Company | Catalog Number | Comments |
| Isoflurane | Halocarbon Products Corporation | NDC 12164-002-25 | 4% |
| aCSF (total:1 L): | |||
| D(+)-Glucose | MERCK | 1.08337.1000 | 10 mM |
| Sodium hydrogen carbonate | MERCK | 1.06329.0500 | 25 mM |
| Sodium chloride | MERCK | 1.06404.1000 | 124 mM |
| (+)-Sodium L-ascorbate, >=98% | SIGMA | A4034-100G | 0.15 g/2 c.c |
| Magnesium sulfate, anhydrous, ReagentPlus | SIGMA | M7506-500G | 2 mM |
| Calcium chloride dihydrate | MERCK | 1.02382.1000 | 2 mM |
| Sodium dihydrogen phosphate monohydrate | MERCK | 1.06346.1000 | 1 mM |
| Potassium chloride | May & Baker LTD Dagenham England | MS 7616 | 4.4 mM |
| (+)-Bicuculline | TOCRIS | 130 | 5 µM in aCSF |
| 4-Aminopyridine | TOCRIS | 940 | 250 µM in aCSF |
| Vibratome | Vibratome | Series 1000 | Block slicing into 500 µm thick slices |
| Multielectrode array (MEA) probes: 6 x 10 planar MEA | Multi Channel Systems | 60MEA500/30iR-Ti-pr MEAS 6x10 | electrode diameter, 30 µm; electrode spacing, 500 µm; impedance, 50 kΩ at 200 Hz |
| Multielectrode array (MEA) probes: 8 x 8 MEA | Ayanda Biosystems | 60MEA200/10iR-Ti-pr MEAS 8x8 | pyramidal-shaped electrode; diameter, 40 µm; tip height, 50 µm; electrode spacing, 200 µm; impedance, 1,000 kΩ at 200 Hz |
| A 60-channel amplifier was used with a band-pass filter set between 0.1 Hz and 3 KHz at 1,200X amplification | Multi-Channel Systems | MEA-1060-BC | |
| MC Rack software at a 10 KHz sampling rate | Multi-Channel Systems | Software for data collect and recordings | |
| control of a pulse generator | Multi-Channel Systems | STG 1002 | |
| slice anchor kits and hold-downs | Warner Instruments | SHD-26H/10; WI64-0250 | |
| Peristaltic Pump-minipuls3 | Gilsom | MINIPULS3 | perfusion rate : 8 ml/min |
| Isolated stimulator | A-M Systems | Model 2100 | intensity of ±350 μA , duration of 200 μsec |
| Tungsten electrode | A-M Systems | 575300 | placed in thalamus |
参考文献
This article has been published
Video Coming Soon
Source: Lu, H. C., et al. Direct-current stimulation and multi-electrode array recording of seizure-like activity in mice brain slice preparation. J. Vis. Exp. (2016).
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。