需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
高吞吐量的单细胞和多细胞微胶囊
摘要
结合单分散细胞和颗粒的惯性顺序降代,我们描述了一种方法封装在一个单一千赫率下降所需的细胞或颗粒数。我们证明效率两次超过无序封装的单粒子和双滴。
摘要
Microfluidic encapsulation methods have been previously utilized to capture cells in picoliter-scale aqueous, monodisperse drops, providing confinement from a bulk fluid environment with applications in high throughput screening, cytometry, and mass spectrometry. We describe a method to not only encapsulate single cells, but to repeatedly capture a set number of cells (here we demonstrate one- and two-cell encapsulation) to study both isolation and the interactions between cells in groups of controlled sizes. By combining drop generation techniques with cell and particle ordering, we demonstrate controlled encapsulation of cell-sized particles for efficient, continuous encapsulation. Using an aqueous particle suspension and immiscible fluorocarbon oil, we generate aqueous drops in oil with a flow focusing nozzle. The aqueous flow rate is sufficiently high to create ordering of particles which reach the nozzle at integer multiple frequencies of the drop generation frequency, encapsulating a controlled number of cells in each drop. For representative results, 9.9 μm polystyrene particles are used as cell surrogates. This study shows a single-particle encapsulation efficiency Pk=1 of 83.7% and a double-particle encapsulation efficiency Pk=2 of 79.5% as compared to their respective Poisson efficiencies of 39.3% and 33.3%, respectively. The effect of consistent cell and particle concentration is demonstrated to be of major importance for efficient encapsulation, and dripping to jetting transitions are also addressed.
Introduction
Continuous media aqueous cell suspensions share a common fluid environment which allows cells to interact in parallel and also homogenizes the effects of specific cells in measurements from the media. High-throughput encapsulation of cells into picoliter-scale drops confines the samples to protect drops from cross-contamination, enable a measure of cellular diversity within samples, prevent dilution of reagents and expressed biomarkers, and amplify signals from bioreactor products. Drops also provide the ability to re-merge drops into larger aqueous samples or with other drops for intercellular signaling studies.1,2 The reduction in dilution implies stronger detection signals for higher accuracy measurements as well as the ability to reduce potentially costly sample and reagent volumes.3 Encapsulation of cells in drops has been utilized to improve detection of protein expression,4 antibodies,5,6 enzymes,7 and metabolic activity8 for high throughput screening, and could be used to improve high throughput cytometry.9 Additional studies present applications in bio-electrospraying of cell containing drops for mass spectrometry10 and targeted surface cell coatings.11 Some applications, however, have been limited by the lack of ability to control the number of cells encapsulated in drops. Here we present a method of ordered encapsulation12 which increases the demonstrated encapsulation efficiencies for one and two cells and may be extrapolated for encapsulation of a larger number of cells.
To achieve monodisperse drop generation, microfluidic "flow focusing" enables the creation of controllable-size drops of one fluid (an aqueous cell mixture) within another (a continuous oil phase) by using a nozzle at which the streams converge.13 For a given nozzle geometry, the drop generation frequency f and drop size can be altered by adjusting oil and aqueous flow rates Qoil and Qaq. As the flow rates increase, the flows may transition from drop generation to unstable jetting of aqueous fluid from the nozzle.14
When the aqueous solution contains suspended particles, particles become encapsulated and isolated from one another at the nozzle. For drop generation using a randomly distributed aqueous cell suspension, the average fraction of drops Dk containing k cells is dictated by Poisson statistics, where Dk = λk exp(-λ)/(k!) and λ is the average number of cells per drop. The fraction of cells which end up in the "correctly" encapsulated drops is calculated using Pk = (k x Dk)/Σ(k' x Dk'). The subtle difference between the two metrics is that Dk relates to the utilization of aqueous fluid and the amount of drop sorting that must be completed following encapsulation, and Pk relates to the utilization of the cell sample. As an example, one could use a dilute cell suspension (low λ) to encapsulate drops where most drops containing cells would contain just one cell. While the efficiency metric Pk would be high, the majority of drops would be empty (low Dk), thus requiring a sorting mechanism to remove empty drops, also reducing throughput.15
Combining drop generation with inertial ordering provides the ability to encapsulate drops with more predictable numbers of cells per drop and higher throughputs than random encapsulation. Inertial focusing was first discovered by Segre and Silberberg16 and refers to the tendency of finite-sized particles to migrate to lateral equilibrium positions in channel flow. Inertial ordering refers to the tendency of the particles and cells to passively organize into equally spaced, staggered, constant velocity trains. Both focusing and ordering require sufficiently high flow rates (high Reynolds number) and particle sizes (high Particle Reynolds number).17,18 Here, the Reynolds number Re =uDh/ν and particle Reynolds number Rep =Re(a/Dh)2, where u is a characteristic flow velocity, Dh [=2wh/(w+h)] is the hydraulic diameter, ν is the kinematic viscosity, a is the particle diameter, w is the channel width, and h is the channel height. Empirically, the length required to achieve fully ordered trains decreases as Re and Rep increase. Note that the high Re and Rep requirements (for this study on the order of 5 and 0.5, respectively) may conflict with the need to keep aqueous flow rates low to avoid jetting at the drop generation nozzle. Additionally, high flow rates lead to higher shear stresses on cells, which are not addressed in this protocol. The previous ordered encapsulation study demonstrated that over 90% of singly encapsulated HL60 cells under similar flow conditions to those in this study maintained cell membrane integrity.12 However, the effect of the magnitude and time scales of shear stresses will need to be carefully considered when extrapolating to different cell types and flow parameters. The overlapping of the cell ordering, drop generation, and cell viability aqueous flow rate constraints provides an ideal operational regime for controlled encapsulation of single and multiple cells.
Because very few studies address inter-particle train spacing,19,20 determining the spacing is most easily done empirically and will depend on channel geometry, flow rate, particle size, and particle concentration. Nonetheless, the equal lateral spacing between trains implies that cells arrive at predictable, consistent time intervals. When drop generation occurs at the same rate at which ordered cells arrive at the nozzle, the cells become encapsulated within the drop in a controlled manner. This technique has been utilized to encapsulate single cells with throughputs on the order of 15 kHz,12 a significant improvement over previous studies reporting encapsulation rates on the order of 60-160 Hz.4,15 In the controlled encapsulation work, over 80% of drops contained one and only one cell, a significant efficiency improvement over Poisson (random) statistics, which predicts less than 40% efficiency on average.12
In previous controlled encapsulation work,12 the average number of particles per drop λ was tuned to provide single-cell encapsulation. We hypothesize that through tuning of flow rates, we can efficiently encapsulate any number of cells per drop when λ is equal or close to the number of desired cells per drop. While single-cell encapsulation is valuable in determining individual cell responses from stimuli, multiple-cell encapsulation provides information relating to the interaction of controlled numbers and types of cells. Here we present a protocol, representative results using polystyrene microspheres, and discussion for controlled encapsulation of multiple cells using a passive inertial ordering channel and drop generation nozzle.
研究方案
本节中的协议描述了利用,特别是获得实验结果提出的材料和设备。需要注意的是可利用替代化学品和设备供应商。
1。设备制造和软光刻技术
标准软光刻技术,21日已在以前的朱庇特的文章特色的数字,22个用于创建聚二甲基硅氧烷(PDMS)粘接到玻璃基板的微网络。除了从主副本SU-8光刻的模具制造,过程可能进行洁净室或洁净罩外;然而,尘埃和微粒仍然应该最小化,以达到一致的结果。
- 在AutoCAD(Autodesk公司)在图1所示,设计了一个微通道模式。聘请第三方制造商(Fineline影像公司)打印一个高分辨率(50,000 dpi)的反麦拉片或石英的渠道是一个黑暗的背景上透明的透明度面具。
- 创建一个副本成型的硅和SU-8光阻主。简言之,旋转SU-8 2050年(MicroChem)负光阻旋转涂布机,以创造一个干净的7.5厘米或10厘米硅片上的厚厚的一层52微米的制造商建议的转。经过软烤,边珠去除紫外线照射,通过接触口罩,接触后烘,发展,和洪水接触,测量的SU-8层,使用的Dektak轮廓(Veeco公司)的实际厚度。磁带准备PDMS的副本成型模具师傅到4“或5”培养皿的底部。
- 混合硅橡胶弹性体弹性体固化剂,在10:1的比例W / W基固化剂(道康宁)的基础上。倒入硅主充分混合的PDMS先导,以创建一个最终层厚度2-3毫米。一个橡胶基地20克,2克固化剂的混合物,是足以应付4“直径表面。
- 放置的肥大ŕ模具和PDMS(Jencons)在真空干燥器的气体固化硅橡胶。使用压力调节器(科尔帕默),缓慢下降超过20分钟-27“汞”汞从0腔表压,以避免过多的泡沫。 -27“汞留在30分钟或直到气泡消失在真空室设备。
- 释放真空,将主模和PDMS为65°C的烘箱中至少四小时(Thermo Scientific的)。该设备可能会留在一夜之间改善固化烤箱。
- 从烤箱取出设备,并允许降温。仔细剪裁的PDMS周围圆形晶圆用一个精确的刀和剥离出的PDMS。 如图1所示,用手术刀切割设备的轮廓。
- 打孔流体端口(每台设备3)在图1所示的三个圆形区域使用活检冲。此设备,使用0.75毫米外径冲(哈里斯)。
- 坚持透明胶带图案的PDMS和剥离面,以消除任何灰尘。为节省成本,但可行的替代传统的氧等离子体仪器,21,22血浆治疗的PDMS和清洁3“×1”玻璃显微镜幻灯片的图案边,使用手持式实验室电晕处理机(电解工艺制品有限公司。)23请注意,此设备应在通风橱或通风良好的地方由于臭氧放电,所有手表和手机应保持至少10英尺的距离。调整最小的火花,达到一个稳定的电晕,电晕放电。慢慢地挥动电极约1/4“以上每面约20秒钟,然后立即把处理后的表面接触,形成了强大的永久债券前返回他们的原生状态的PDMS表面。
- 设备上放置一块金属板,在阴凉烤箱,设定烤箱至120°C,烤一夜之间完成粘接的PDMS在这种高温烘烤,T返回到其原有的疏水状态。24他的玻璃表面的通道也将呈现疏水由于薄的疏水层的玻璃上沉积。另外,疏水涂层,如Aquapel(PPG工业公司)可能会被注入流体用1毫升注射器和针头端口12。谨慎但坚定地注入清除空气流体端口的PDMS没有打破玻璃债券Aquapel 。关闭任何多余Aquapel的边擦,以避免任何可能堵塞后,干燥的渠道存款,积极地重复对所有进口和出口的空气净化。
2。样品制备
- 根据您所选择的细胞类型的既定程序,准备细胞培养。 8-15微米的颗粒或细胞在这项研究中所使用的特殊设备,应充分订购封装。较小或较大的细胞类型,可能需要改变的重点通道的尺寸,以达到足够的再带够 。对于我本文thod示范结果表明,9.9微米的聚苯乙烯微球(G1000的Thermo Scientific的)被用作细胞的代理人。
- 准备通过温和的混合水粒子或细胞悬液。当使用电池或聚苯乙烯颗粒,浓度控制是必要的( 见图4),以达到理想的有序封装。使用以前的数据为指导,计算出所需的细胞或粒子浓度的基础上有序的列车间距和微通道尺寸为:一个细胞或颗粒每预期的纵向列车间隔时间聚焦通道截面积。如果股票浓度(1%W / W)是不够的,增加浓度(1.5%W / W)股票样本轻轻离心,除去上清液,和再悬浮旋涡混合,或温和的混合颗粒当使用电池。准备足够的量,占所需收集的数量和运行时间与FLOW调整。
- 两种细胞和聚苯乙烯颗粒有一个特定的重力大于一。虽然没有在这个协议表明,持续多少分钟到几个小时的秩序的长期实验,浮力匹配的解决方案,通过添加溶质,如氯化钙颗粒或细胞OptiPrep(Sigma-Aldrich公司)。
- 准备10毫升样品的连续氟碳油相混合氟碳油FC-40(3M)和聚醚-聚乙二醇嵌段共聚物表面活性剂(2.5%W / W)25(飞雨)在15 mL离心管。另外,轻矿物油,(交汇处过程化学品),可以利用ABIL-EM 90表面活性剂(2.5%W / W)(赢创高施米特公司)。
3。实验装置
- 倒置光学显微镜(Axio上观察,蔡司)和高速摄影机(幻影V310,视觉研究)的电源。重点和检查和杂物堵塞的渠道,或通过手动移动设备使用电动显微镜阶段。当液体流经一些小碎片可能会被推。对于大型的碎片或明显的木屐,选择另一个设备上的通道,在重点通道的碎片可以订购质量显着降低。请注意,木屐通常可以根据流量按上述钝镊子受灾地区的PDMS表面上牢固删除。
- 切水入口的长度为(0.01“ID/0.03”外径,聚乙烯)PVC管材,进油口,和乳液插座。为了尽量减少死体积,减少刚够油管,以达到从注射器泵在显微镜阶段。切断油管两端45°角,以方便插入到流体港口。
- 使用镊子,按适应管在第1步砸出流体端口结束,然后按各自的水和油口管(无粘合剂必要)的自由端放入两个30号钝尖不锈钢注射器针头(SmallParts) 。放入废物ŕ的出口管eservoir。该管稍后将搬进一家集水库。
- 移动设备和连接管显微镜的阶段,调整,集中在设备的使用提供客观的喷嘴(20倍,本实验所用)。调整ķhler照明和其他显微镜设置为最佳录音。
- 混合水相和油相的解决方案,准备在第2步3毫升注射器(BD),填补了1毫升注射器(BD)。请注意,任何体积的注射器,可使用取决于所需的运行时间和最小化任何搏动,应慎重选择。倾斜注射器垂直轻弹气泡移动到注射器插座。慢慢压低柱塞足以推动注射器尖端的空气。拿着注射器垂直,连接到步骤3.3的设备已经连接到各自的注射器针头的注射器。压下柱塞强行通过注射器针头死体积的空气,直到流体是p通过油管设备几乎ushed。牢固安装注射器,注射器泵(NEXUS 3000 Chemyx)和从事柱塞块。第二注射器重复的连接,并安装到第二个注射泵。
- 每个注射泵的权力和程序,使用泵制造商的协议。设置初始流量为 Q 油 = 50μL/分钟和 Q AQ = 5μL/分钟,分别为油相和水相中。启动泵。
- 等待每个流体进入设备填补渠道,推动了其余的死气。这可能需要几分钟。如果有大量的空气入口管,临时增加每个流量,直到空气被驱逐。不增加流量率如此之高,发生大的压力的通道,可能会导致硅橡胶,玻璃债券失败。
- 使用初始流速,观察滴在喷嘴形成(结果显示在这里:20X magnificatioN,帧频21005 FPS,曝光3微秒)。减少相机领域的观点只喷嘴,以最大限度地提高帧速率和减少内存需求,如果可能的话。捕获样本的影片,并确认采样率是足够的,以避免走样。
- 为了避免喷射( 见图2),开始与低水的流速。慢慢增加水的流速,观察颗粒订货,只要流量的增加水溶液通道。
- 如果颗粒物浓度太低提供相对较少的“失踪”的粒子和样品不匹配浮力的火车,身体倾斜,向注射泵的注射器出口提供的粒子向注射器出口的逐步解决。这种方法被证明在视频协议。定期注射器沿其轴线旋转,也可减少不受欢迎的沉淀。
- 一旦发生足够的顺序,调整油流量调整代频率和滴的大小。可以计算平均下降量,利用水的流速由下拉代视频捕捉测量频率划分。反复调整两个流率,以达到预期的包封率和降量。
- 一旦稳定有序封装证实,从废物水库出口油管移动到一个集合水库或送入另一个随后的测试设备。
- 确定集合时间,根据所需数量的飞沫和计算的发电频率。
- 记录包含0,1,2,...,N粒子使用或吸取样品收集乳液检查或者降代视频结果量化效率下降的一小部分。
4。代表结果
结果,提出了实现同时控制单粒子和控制的双粒子封装( 图3)。通过削减FC-40在半油流率,单粒子封装成为两粒子封装。相反,我们可以增加水的流速,更迅速地提供颗粒的喷嘴,但我们也将增加喷射水流的风险。在图3的直方图提出该两宗案件的每一滴水颗粒小数,随着泊松统计的比较。零颗粒,偶尔滴,主要是由于“失踪”在有序的列车颗粒,而那里有从局部高颗粒浓度和颗粒有时朝两个垂直聚焦位置迁移的结果比预期的更封装颗粒的案件。请注意没有利用浮力匹配,如在第2节所述。相反,注射泵,身体倾斜,允许向注射器出口的粒子沉降,导致高浓度的运行过程中的颗粒。
说明需要适当的颗粒和细胞浓度的实验运行, 如图4所示。没有充分的顺序,颗粒秩序的本地化团体和封装,但很多滴,无颗粒。直方图显示所需的颗粒封装包封率下降。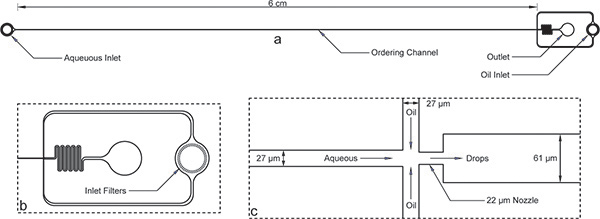
图1。封装设备。 a)整体设备入口,出口和长期订购通道。设备高度为52μm和订购的通道宽度为27微米。 B)水和油入口有大的杂物过滤器订购通道宽度放大的进油口秩序的差距。三)扩大喷嘴视图显示等通道宽度由22微米的喷嘴,突然扩展到更宽的61微米通道收缩水和油的渠道,其次为27微米。请注意,这里显示设备的尺寸已经验证,使用后微细1轮廓,从面具的标称尺寸略有不同。订购通道和喷嘴的真实形象可在网上作为参考图1 。 AutoCAD的掩码文件也被列入网上这个手稿的补充。
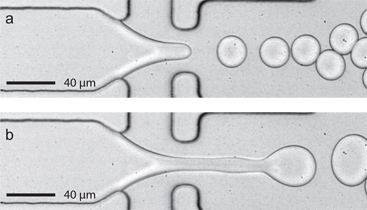
图2。一个滴水喷射过渡使用更广泛的设备(高80μm宽×22微米)的迟滞。一)常数的FC-40流量(问油 = 45μL/分钟),采用水溶液流量 Q AQ = 8μL/ min的稳步下降的形成发生在10 kHz。由于水的流速缓慢上升到10Μ升/分钟,水流体流喷射触发。 b)当流量返回到8μL/ min的喷射持续。注意稳步下降形成简要暂停水流量泵(1秒的停顿是典型的),可以重新建立。
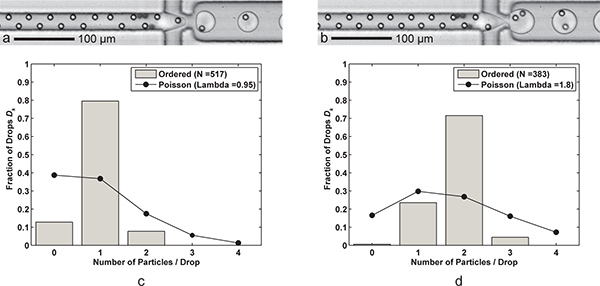
图3。单粒子和双封装。掉落的形成与每下降一个细胞()Q 油 = 60μL/分钟,问AQ = 9μL/分钟)下降了6.1 kHz的生成率,平均降幅大小24.4 PL,和ðK = 79.5%,P K = n个样本大小为83.7%(λ= 0.95)D = 517滴和N P = 491颗粒。 二)与两个细胞下降形成一种单细胞的采集效率每一滴水,实现简单的FC-40 流量 Q 石油减少30μ升/分钟。较大(39.8 PL)下降3.8千赫的速度形成两个细胞的采集效率ðķ= 71.5%,P K = 79.5%(λ= 1.80)为n 的样本大小= 383滴和 n P = 689粒子。CD)两个直方图比较下降封装颗粒效率D的定购单和双粒子与泊松统计(随机封装)封装ķ。请注意,这两种情况下,完全有序,交替颗粒在流动方向的粒子间距约17-18微米。补充视频显示单粒子和双封装可在网上点击这里查看补充电影3A 点击这里查看补充电影3B 。

图4。浓度的包封率有很大影响。A)随着浓度的降低,充分顺序不会发生,因此,“洞”在列车的出现,留下一些下降少于预期颗粒。 二)直方图显示的效率下降( ðķ= 55.9%,P K = 70.9%,为两粒子的封装,由于较低的λ值= 1.57那里有几乎同样多的单粒子下降,因为有双粒子滴)。这个数字结果从 Q 油 = 30μL/ min的和 Q AQ = 9μL/ min的相同流量条件下, 图3b。一位代表补充视频可在网上点击这里查看补充电影4 。
讨论
尽管订货度比较高,并非所有滴将包含适当数量的粒子或细胞。包封可以封装成为在滴除以他们的总人数所需占用的细胞或颗粒的数量计算。无论是从一个自动化的高速视频算法或从成像收集乳液样品,可以得到这些原始数据。这可以相比下降封装在一个包含k粒子和分数,将包含ķ颗粒含滴ðK粒子的P K的分数。从图3中 ,单和双颗粒封装效率优于随机包?...
披露声明
乙脑是在这个手稿中使用的技术为基础的待批专利的发明者。
致谢
我们感谢飞雨技术,聚醚,聚乙二醇表面活性剂在这项研究中使用的样品,我们感谢微机电资源中心(穆罕默德·碳粉,主任),用于创建的PDMS通道副本硅片模具。
材料
| Name | Company | Catalog Number | Comments |
| 试剂名称 | 公司 | 目录编号 | 评论 |
| AutoCAD的 | 欧特克 | ||
| 透明面具 | fineline成像公司 | ||
| SU-8胶 | MicroChem | 2050 | |
| dektak轮廓 | Veeco公司 | ||
| 培养皿 | 屋宇署猎鹰 | 351058 | |
| 硅橡胶硅橡胶套件 | 道康宁公司 | sylgard 184,材料编号(240)4019862 | |
| 真空干燥器 | jencons | 250-030 | |
| 真空泵 | 阿尔卡特高真空技术 | 2010的C2 | |
| 真空调节器 | 科尔 - 帕默 | EW-00910-10 | |
| 烤箱 | Thermo Scientific的 | 林德伯格蓝M,OV800F | |
| 活检冲床,0.75毫米 | 哈里斯 | 统一的核心15072 | |
| 实验室电晕处理机 | 电,工艺制品有限公司 | BD-20AC,12051A的SKU | |
| 载玻片 | 金印 | 3010 | |
| aquapel | PPG工业公司 | 替代战略 | |
| 聚苯乙烯微球,9.9微米 | 热 | G1000的 | |
| OptiPrep | Sigma-Aldrich公司 | D1556 | 没有表现出 |
| 鲁尔 - 乐注射器 | 屋宇署 | 1毫升:309628 3毫升:309585 | |
| FC-40氟碳油 | 3M公司 | Sigma Aldrich公司,F9755 | |
| 聚醚 - 聚乙二醇含氟表面活性剂 | 飞雨技术 | ||
| 轻质矿物油 | 公共交通交汇处过程化学品 | 08042-47-5 | 替代战略 |
| 矿物油表面活性剂 | 赢创高施米特公司 | ABIL的EM 90 | 替代战略 |
| 聚乙烯聚氯乙烯管 | SmallParts | TGY-010 | |
| 30计鲁尔乐注射器针头,1/2“ | SmallParts | ñ-301PL-C的 | |
| 倒置显微镜 | 卡尔蔡司影像 | AXIO Observer.Z1 | |
| 高速摄影机 | 视觉研究 | 幻影V310 | |
| 注射泵(2) | chemyx公司 | NEXUS 3000 | |
| 硅油 | 道康宁 | 200液,10 CST | 乳化存储可选 |
参考文献
- Zagnoni, M., Lain, G. L. e., Cooper, J. M. Electrocoalescence mechanisms of microdroplets using localized electric fields in microfluidic channels. Langmuir : the ACS journal of surfaces and colloids. 26, 14443-14449 (2010).
- Niu, X. Z., Gielen, F., Edel, J. B., deMello, A. J. A microdroplet dilutor for high-throughput screening. Nat. Chem. 3, 437-442 (2011).
- Vincent, M. E., Liu, W., Haney, E. B., Ismagilov, R. F. Microfluidic stochastic confinement enhances analysis of rare cells by isolating cells and creating high density environments for control of diffusible signals. Chemical Society reviews. 39, 974-984 (2010).
- Huebner, A. Quantitative detection of protein expression in single cells using droplet microfluidics. Chemical communications. , 1218-1220 (2007).
- Love, J. C., Ronan, J. L., Grotenbreg, G. M., van der Veen, A. G., Ploegh, H. L. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nature biotechnology. 24, 703-707 (2006).
- Bradshaw, E. M. Concurrent detection of secreted products from human lymphocytes by microengraving: Cytokines and antigen-reactive antibodies. Clin. Immunol. 129, 10-18 (2008).
- Liu, W. S., Kim, H. J., Lucchetta, E. M., Du, W. B., Ismagilov, R. F. Isolation, incubation, and parallel functional testing and identification by FISH of rare microbial single-copy cells from multi-species mixtures using the combination of chemistrode and stochastic confinement. Lab on a chip. 9, 2153-2162 (2009).
- Boedicker, J. Q., Li, L., Kline, T. R., Ismagilov, R. F. Detecting bacteria and determining their susceptibility to antibiotics by stochastic confinement in nanoliter droplets using plug-based microfluidics. Lab on a chip. 8, 1265-1272 (2008).
- Koster, S. Drop-based microfluidic devices for encapsulation of single cells. Lab on a chip. 8, 1110-1115 (2008).
- Kelly, R. T., Page, J. S., Marginean, I., Tang, K., Smith, R. D. Dilution-free analysis from picoliter droplets by nano-electrospray ionization mass spectrometry. Angew Chem. Int. Ed. Engl. 48, 6832-6835 (2009).
- Hong, J., deMello, A. J., Jayasinghe, S. N. Bio-electrospraying and droplet-based microfluidics: control of cell numbers within living residues. Biomedical materials. 5, 21001 (2010).
- Edd, J. F. Controlled encapsulation of single-cells into monodisperse picolitre drops. Lab on a chip. 8, 1262-1264 (2008).
- Anna, S. L., Bontoux, N., Stone, H. A. Formation of dispersions using "flow focusing" in microchannels. Applied Physics Letters. 82, 364 (2003).
- Utada, A., Fernandez-Nieves, A., Stone, H., Weitz, D. Dripping to Jetting Transitions in Coflowing Liquid Streams. Physical Review Letters. 99, (2007).
- Chabert, M., Viovy, J. L. Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells. Proceedings of the National Academy of Sciences of the United States of America. 105, 3191-3196 (2008).
- Segrí, G., Silberberg, A. Radial Particle Displacements in Poiseuille Flow of Suspensions. Nature. 189, 209-210 (1961).
- Carlo, D. D. i. Inertial microfluidics. Lab on a chip. 9, 3038-3046 (2009).
- Carlo, D. D. i., Edd, J., Humphry, K., Stone, H., Toner, M. Particle Segregation and Dynamics in Confined Flows. Physical Review Letters. 102, (2009).
- Humphry, K. J., Kulkarni, P. M., Weitz, D. A., Morris, J. F., Stone, H. A. Axial and lateral particle ordering in finite Reynolds number channel flows. Physics of Fluids. 22, 081703 (2010).
- Lee, W., Amini, H., Stone, H. A., Carlo, D. D. i. Dynamic self-assembly and control of microfluidic particle crystals. Proceedings of the National Academy of Sciences of the United States of America. 107, 22413 (2010).
- Duffy, D. C., McDonald, J. C., Schueller, O. J. A., Whitesides, G. M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane. Anal. Chem. 70, 4974-4984 (1998).
- Kotz, K., Cheng, X., Toner, M. PDMS Device Fabrication and Surface Modification. J. Vis. Exp. (8), e319 (2007).
- Haubert, K., Drier, T., Beebe, D. PDMS bonding by means of a portable, low-cost corona system. Lab on a chip. 6, 1548-1549 (2006).
- Hatch, A. C. 1-Million droplet array with wide-field fluorescence imaging for digital PCR. Lab on a chip. , 3838-3845 (2011).
- Holtze, C. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab on a chip. 8, 1632-1639 (2008).
- Garstecki, P., Stone, H., Whitesides, G. Mechanism for Flow-Rate Controlled Breakup in Confined Geometries: A Route to Monodisperse Emulsions. Physical Review Letters. 94, (2005).
- Garstecki, P., Fuerstman, M. J., Stone, H. A., Whitesides, G. M. Formation of droplets and bubbles in a microfluidic T-junction-scaling and mechanism of break-up. Lab on a chip. 6, 437-446 (2006).
- Nie, Z. Emulsification in a microfluidic flow-focusing device: effect of the viscosities of the liquids. Microfluidics and Nanofluidics. , (2008).
- Holt, D. J., Payne, R. J., Chow, W. Y., Abell, C. Fluorosurfactants for microdroplets: interfacial tension analysis. Journal of colloid and interface science. 350, 205-211 (2010).
- Holt, D. J., Payne, R. J., Abell, C. Synthesis of novel fluorous surfactants for microdroplet stabilisation in fluorous oil streams. Journal of Fluorine Chemistry. 131, 398-407 (2010).
- Hatch, A. C., Fisher, J. S., Pentoney, S. L., Yang, D. L., Lee, A. P. Tunable 3D droplet self-assembly for ultra-high-density digital micro-reactor arrays. Lab on a chip. 11, 2509-2517 (2011).
- Baret, J. C. Surfactants in droplet-based microfluidics. Lab on a chip. 12, 422-433 (2012).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。