Method Article
关于功能研究的三维人体肺组织模型
摘要
Human tuberculosis infection is a complex process, which is difficult to model in vitro. Here we describe a novel 3D human lung tissue model that recapitulates the dynamics that occur during infection, including the migration of immune cells and early granuloma formation in a physiological environment.
摘要
结核病(TB)仍然占据着一个重大威胁人们的健康全世界,并且有必要对成本效益的,但可靠的模型帮助我们理解疾病机制和推动新的治疗选择的发现,在 体外细胞单层培养或共培养物缺乏所述三维(3D)环境和组织反应。在此,我们描述了一个创新的人的肺组织,它有希望成为一个有效的工具,研究发生感染结核分枝杆菌(结核杆菌 )在复杂事件的体外模型 。三维组织模型由组织特异性上皮细胞和成纤维细胞,它们是在胶原蛋白上的多孔膜的顶部上的基质中培养的。一旦暴露在空气中,上皮细胞分层和分泌粘液的顶侧。通过将人的主要巨噬细胞感染了M.结核病到组织模式升,我们已经表明,免疫细胞迁移至感染组织并形成结核病肉芽肿早期阶段。这些结构概括人类结核病的肉芽肿,这是根本不同的或广泛使用的实验动物模型中没有普遍观察到的显着特点。此器官培养方法使3D可视化和健壮定量分析,提供对宿主细胞 - 病原体相互作用的空间和时间特性枢轴信息。总之,肺组织模型提供了生理相关的组织微环境对结核病的研究。因此,肺组织模式有两个基本机理和应用研究的潜在影响。重要的是,该模型允许除了个别的细胞类型,这由此扩大其使用用于建模的各种影响肺部感染性疾病或操纵。
引言
在人类中,反应感染,组织发炎,细胞招募,组织重塑和组织稳态的调节是涉及不同的细胞类型的复杂的事件。因此,这些过程最好研究了在局部组织环境中。先前,这已经用实验动物模型中主要是不可能的。但是,广泛使用的实验动物容纳许多限制,因为他们往往病原体响应以不同的方式比人类,也显示不同的病程1。 体外肺组织模型甲人持有的可能性,研究在人肺特异性免疫应答。
人类结核感染(TB)是主要的疾病影响肺部。 结核分枝杆菌(结核分枝杆菌 ),结核病的病原体,到达经由被运送到肺泡空间,其中该细菌通过肺dendri吞没气溶胶小滴的肺抽动细胞和肺泡巨噬细胞,以感染2,3先天免疫应答的一部分。病原体的吞噬导致吞噬小体中的bug条块分割,最好的结果,在中和杀害的病原体引起的吞噬。达暴露于分枝个体的50% 结核被认为是能够通过先天免疫反应4以清除感染。感染的其他成果是间隙由适应性免疫系统在后一阶段,潜伏感染或在最坏的情况下慢性活动性疾病5。
以前没有出现过的体外组织模型为人类结核病的研究。人巨噬细胞或其它外周血细胞的单细胞培养物常常被用来6,7。这种方法的缺点是,它们不能反映不同的细胞类型一起操作以肺组织暴露到 M的动态肺结核 。因此,存在需要一种体外模型 ,以便能够对结核病执行功能和机理研究。基于细胞的在本文所述体外人肺组织模型最初由我们的组对树突状细胞功能8研究建立的。我们已经适应了这种方法用于结核病的研究。
这里介绍的人肺组织模型由组织特异性上皮细胞和成纤维细胞8。这些细胞在胶原蛋白上的Transwell小插入物和形式的结构类似于正常人肺组织(图1)的多孔膜的顶部上的基质中培养。当暴露于空气中的细胞开始分泌粘液在根尖侧 8。通过植入人体主要的巨噬细胞感染了M.结核病的模型,我们已经观察到的免疫细胞在组织中如何迁移并形成结核病肉芽肿9的早期阶段。这是人类第一次组织模型DESCRIBED结核病和它构成一个行之有效的手段研究先天免疫反应结核病和肺部等疾病。到目前为止,我们已经使用只单核细胞和巨噬细胞作为免疫细胞中的模式,但复杂的水平可以增加通过包含额外的相关细胞类型。
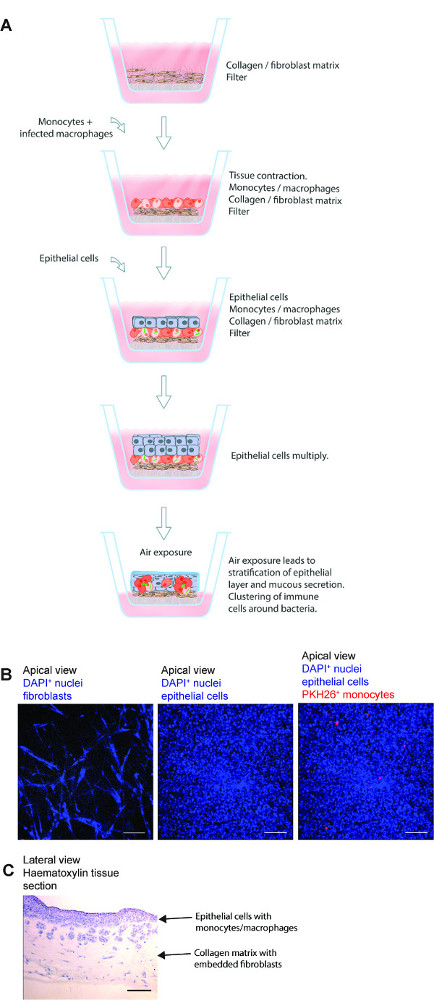
图肺组织模型1.纲要。 (A),该模型是由人的肺特异性上皮细胞,M。结核感染的原发性巨噬细胞和红色染料标记的单核细胞接种于准备在Transwell滤膜胶原嵌入式成纤维细胞。曝光的组织模型的空气由上皮发起生产细胞外基质蛋白,粘液分泌和分层。因此,开发的3D组织模型是研究M.一个有用的工具结核感染在CLO环境sely类似于一个人肺。(B)的在所述组织模型的制备的不同步骤代表性显微图像。(C)的肺模型组织切片的完全结构。秤- 100微米请点击此处查看该图的放大版本。
研究方案
注:从在林雪平大学医院的血库购买健康匿名献血者的外周血中,瑞典作为免疫细胞的来源,这项研究。这个协议设计24毫米6孔板插入。直接适应等众多格式,不推荐,因为组织合同范本垂直和水平的发展过程中。
1.准备的细菌/细胞系的材料,媒介和文化
- 文化的细菌:
- 生长分枝杆菌菌株M.结核分枝杆菌H37Rv携带pFPV2质粒以组成型表达绿色荧光蛋白(GFP),在含有0.05%的Middlebrook 7H9培养基吐温-80,0.5%甘油,卡那霉素(20微克/毫升),并补充有米德尔白蛋白,右旋糖和过氧化氢 酶的富集(的Middlebrook ADC富集),在37℃,5%CO 2的7-10天。
注:所有的实验步骤小号涉及活毒力M.杆菌菌株应以BSL-3设施来进行。
- 生长分枝杆菌菌株M.结核分枝杆菌H37Rv携带pFPV2质粒以组成型表达绿色荧光蛋白(GFP),在含有0.05%的Middlebrook 7H9培养基吐温-80,0.5%甘油,卡那霉素(20微克/毫升),并补充有米德尔白蛋白,右旋糖和过氧化氢 酶的富集(的Middlebrook ADC富集),在37℃,5%CO 2的7-10天。
- 制备1×Dulbecco氏改良的Eagle氏培养基(DMEM)的完全培养基(补充有1mM丙酮酸钠,2mM的L-谷氨酰胺,100U / ml青霉素,100微克/ ml链霉素,10mM的HEPES,0.1mM非必需氨基酸和10 %热灭活胎牛血清(FBS))。还准备抗生素的DMEM完全培养基。
- 制备1×极限必需培养基(MEM)完全培养基(1mM丙酮酸钠,2mM的L-谷氨酰胺,100U / ml青霉素,100微克/ ml链霉素,10mM的HEPES,0.1mM非必需氨基酸和10%热灭活的小牛血清(FBS))。
- 制备的纤连蛋白/胶原涂层的烧瓶(总共10ml)中:
- 吸管8.8毫升无菌1×磷酸盐缓冲盐水(PBS)到干净的管中。加入1毫升牛血清白蛋白(1毫克/毫升),加入100μl的I型牛胶原(3毫克/毫升)和100微升重组胡人纤连蛋白(1毫克/毫升)。
- 通过转动管上下颠倒5次混合溶液。烧瓶涂覆有纤连蛋白/胶原溶液(1ml的T-25和2ml的T-75烧瓶中)。给它O / N在37℃。温育后,除去溶液,并存储涂层烧瓶在RT。
注意:将收集的溶液可以储存在4℃和重复使用三次。存储为2周以上可能使液体变成褐色(晶体形成),其中,在它必须被丢弃。
- 文化成纤维细胞:
- 在37℃下在5%CO 2生长和维持MRC-5,(从一个14周龄的雄性胎儿正常肺组织衍生的人肺成纤维细胞株),在完全DMEM。使用成纤维细胞传代24-26和生长,直到70-80%汇合。
注:在通道> 30 MRC-5株往往失去形态,不推荐用于组织模型的使用。
- 在37℃下在5%CO 2生长和维持MRC-5,(从一个14周龄的雄性胎儿正常肺组织衍生的人肺成纤维细胞株),在完全DMEM。使用成纤维细胞传代24-26和生长,直到70-80%汇合。
- 培养上皮细胞的:
- 获取16HBE14o-(16HBE),永生化人支气管上皮细胞,保留正常的人呼吸道上皮细胞的分化形态和功能线,(这是从迪特尔Gruenert博士,锡安山癌症中心,美国加州大学旧金山礼物,美国10)。培养16HBE细胞中纤连蛋白/胶原包被烧瓶中并保持细胞在完全MEM在5%CO 2在37℃。
- 5X DMEM的制备
- 通过将13.4克的DMEM粉末和3.7克碳酸氢钠在150毫升无菌蒸馏水制备5倍的DMEM。调整培养基的pH至7.3,补足体积200毫升,用0.22μm的膜过滤器过滤。收集在无菌容器中的过滤介质并储存直到使用在RT。
2.制备胶原蛋白包埋成纤维细胞
- FBS和L-谷氨酰胺的在37℃水浴中解冻冷冻的等分试样。解冻后保持样品在冰上。放置碳酸氢钠(71.2毫克/毫升)和庆大霉素(50毫克/毫升)在4℃下。预冷的50ml离心管中和10毫升无菌移液管至4℃。
注:使用(除5倍DMEM)所有材料都是冰鲜的,使用前冰和所有步骤都在冰上进行。 I型牛胶原蛋白(1.1毫克/毫升)必须保持冷,因为这样可以防止胶原蛋白凝固。 - 准备成纤维细胞:
- 暖胰蛋白酶在37℃水浴中孵育足够的量与肺癌的成纤维细胞(MRC-5)的细胞在5%CO 2的10分钟,在37℃。通过添加1X DMEM完全中和胰蛋白酶。吸出细胞悬浮液和离心机在300×g离心5分钟。吸出上清液并将细胞重新悬浮在2.3×10 5个细胞/ ml在DMEM完全。放置在冰上的细胞,直到准备使用。
- 制备预混:
- 以下内容添加到标有"预混合"的管子; 395微升5倍的DMEM,40微升L-谷氨酰胺,120微升碳酸氢钠(71.2毫克/毫升),440微升FBS,5微升庆大霉素(50毫克/毫升),总成交量1000微升,然后漩涡预拌匀装上冰。
注:给出的卷是一个24毫米6孔培养插入。计算所需的刀片总数的特定量,但添加一个额外的,以确保,足够预混制备。
- 以下内容添加到标有"预混合"的管子; 395微升5倍的DMEM,40微升L-谷氨酰胺,120微升碳酸氢钠(71.2毫克/毫升),440微升FBS,5微升庆大霉素(50毫克/毫升),总成交量1000微升,然后漩涡预拌匀装上冰。
- 准备细胞胶原混合:
- 为每一种文化加入1毫升细胞胶原混合物。以下内容添加到一个50毫升的锥形管冰在给定的顺序; 686微升1.1毫克/毫升的胶原,加入250μl预混合和64微升1×完全DMEM至1000微升的总体积。拌匀,确保无气泡的解决方案。工作速度快,并添加在管的壁的胶原以避免气泡。
- 加入1毫升无细胞层混合物与所述插入放置在6孔板中。不要任何介质添加到孔插入外部。于cubate用于在37℃培养箱中30分钟。确保无细胞混合物覆盖整个插入件而没有任何气泡。
- 准备细胞胶原蛋白的混合物:
- 混合蜂窝层的成分在50ml锥形管中保持在冰上以下列顺序; 2 ml的胶原蛋白,615微升预混合,58微升1×完全DMEM和327微升细胞悬浮液肺成纤维细胞(MRC-5),以总体积补足至3000微升。每一种文化需要3毫升细胞胶原蛋白的混合物。
关键的一步:确保除了细胞悬液之前,请仔细混合的胶原蛋白和预混料。这将中和胶原的pH,以避免对成纤维细胞的毒性作用。 - 添加蜂窝层(3毫升)对无细胞胶原层的顶部,并培育2小时在37℃培养箱。工作速度快,并添加在管的壁的胶原以避免气泡。
- 聚合后,加2ml的DMEM完全为t他的6孔板(插入件下)的底部,并培育24小时。
注意:如果聚合并没有出现,丢弃含成纤维细胞胶原基质的板插入和启动一遍。最可能的原因是,除了或以上列出的试剂之一不正确体积的误差。
- 混合蜂窝层的成分在50ml锥形管中保持在冰上以下列顺序; 2 ml的胶原蛋白,615微升预混合,58微升1×完全DMEM和327微升细胞悬浮液肺成纤维细胞(MRC-5),以总体积补足至3000微升。每一种文化需要3毫升细胞胶原蛋白的混合物。
的成纤维细胞胶原基质的3连续培养
- 使用干净镊子小心地提起插入物和从井的底部吸出培养基。加2ml完全DMEM到井后跟的底部由插入件在2 ml的完全DMEM。避免引入气泡插入件下,因为这将防止外和内室之间的营养物质的扩散。除去气泡用枪头。
- 大约5-7天更换培养基(内,插入以下)每两天和文化。小心从插入取出媒体。为了避免CONTA克拉与成纤维细胞的胶原基质,用干净的镊子稍微倾斜插入件和从所述插入件壁吸媒体。
关键步骤:在胶原基质成纤维细胞应该得到一个细长的表型,和重塑胶原,然后合同。在约5-7天,矩阵已签约,以在插入件的中心的平台(10-14毫米直径)。收缩矩阵是准备用于下一步骤中使用。以获得矩阵的均匀收缩,关键的是成纤维细胞与胶原播种(步骤2.6.1)之前充分混合。
4.播种免疫细胞(感染/未感染的单核细胞 - 巨噬细胞混合物)
注意:下面的实验步骤涉及毒性分枝杆菌,因此,必须在一个BSL-3设施来进行。
- 制备初级单核细胞和巨噬细胞的:
- 使用establi从供体血液分离外周血单核细胞棚协议。隔离的单核细胞上的同一天,建立组织模型和培养和感染分枝前分化成巨噬细胞为大约7天后肺结核。
注意:这将确保两个巨噬细胞和收缩成纤维细胞的胶原基质是7天后使用。也分离,将连同感染的巨噬细胞中加入新鲜的单核细胞。
- 使用establi从供体血液分离外周血单核细胞棚协议。隔离的单核细胞上的同一天,建立组织模型和培养和感染分枝前分化成巨噬细胞为大约7天后肺结核。
- M的准备结核感染的巨噬细胞
- 收获培养的细菌,洗用1×PBS含有0.05%Tween-80,重悬在无抗生素的完全DMEM,通过截短无菌27G的针以分散细菌团块并测量光密度。
注意:预确定M.结核菌落形成在实验室光密度单位当量。这将使用于感染细菌的数量的估计值。 - 孵育巨噬细胞4小时与微米。结核病在感染复数(MOI)10)。感染后,洗3次用PBS洗涤,去除细胞外的细菌。用同样的方式,但没有M.培养未感染的巨噬细胞结核病 ,作为对照。
- 通过处理分离从培养板中的巨噬细胞与2mM EDTA的10分钟,在37℃,并再悬浮的细胞在不含抗生素的完全DMEM。
- 收获培养的细菌,洗用1×PBS含有0.05%Tween-80,重悬在无抗生素的完全DMEM,通过截短无菌27G的针以分散细菌团块并测量光密度。
- 单核细胞的标签
注意:分离单核细胞的和标签可以在BSL-2工作台来进行,然后考虑到BSL-3设施,以便进一步处理。- 染色新鲜制备的单核细胞(2×10 7细胞)中的2μMPKH26红色染料5分钟的最终浓度,根据制造商的说明。洗涤3x和重悬细胞与无抗生素的完全DMEM以1×10 7细胞/ ml的密度。
- 此外免疫细胞的成纤维细胞胶原基质
- 一个压脚提升5-7天的成纤维细胞的胶原基质的培养,从外部和内部腔室吸出培养基和新鲜不含抗生素的DMEM完全到外室中添加1.5毫升
- 与MO的比率制备的标记的单核细胞 - 巨噬细胞(未感染/感染)的混合物:MQ(5:1)在50μlDMEM完全。 50000的巨噬细胞,以25万标有单核细胞。
- 添加50μl的MO:MQ混合物到成纤维细胞的胶原基质并孵育1小时,在5% 的 CO 2在37℃。孵育后,轻轻地加2ml培养基到插入并孵育另外24小时,在5% 的 CO 2在37℃。
注:由于加入细胞松散地附着,另外的媒体应当由刀片的墙壁上轻轻加入慢。
5.播种肺上皮细胞(16HBE)
注意:下面的步骤必须在BSL-3设施来进行。
- 种子肺êMQ-成纤维细胞的胶原层:在MO上面pithelial细胞(16HBE)。执行此,第一离解16HBE细胞从烧瓶中通过用胰蛋白酶处理(如在2.2.1。),重悬在4×10 6个细胞/抗生素的DMEM毫升。
- 内部和插入件的外吸去培养基。然后加入1.5 ml的不含抗生素的DMEM完全在井的插入件外底部。
- 加入50微升16HBE的对免疫细胞的成纤维细胞的胶原基质的顶部。留在机罩2分钟,并在37℃培养箱,用5%的CO 2孵育1小时。温育后,在37℃下3天添加轻轻2毫升不含抗生素的DMEM的插入物和培养内完成。培养步骤促进上皮细胞的组织模型的增殖。
注:由于加入细胞松散地附着,另外的媒体应该是缓慢和轻柔的,通过插入件的壁滑动。
6.空中曝光的3D隆型号
注意:在后第5天加入感染的巨噬细胞,组织模型是空气暴露和随后的步骤必须在BSL-3设施来进行。
- 内部和插入件的外吸去培养基。
注:在这一步骤的上清液可以收集用于检测分泌的因子。离心机,无菌过滤并储存上清液在-70℃下。 - 添加1.8 ml的不含抗生素的DMEM完全在外部腔室,并培育在5%CO 2,37℃培养箱内培养2天。不要插入中添加培养基。
注:空气提升组织模型的便于分层上皮和粘液分泌,它提供了强度到组织和生理相似人肺组织的形成。
7.收获和安装3D肺组织模型
注意:下面的步骤必须在BSL-3设施来进行。
- 在受感染的巨噬细胞7天后植入,组织模型是准备收割。完全从组织模型中取出培养基。修复组织模型,用4%多聚甲醛在黑暗中在RT下30分钟。此步骤杀死细菌并固定的组织/细胞的形态,以便进一步处理。
- 使用手术刀,从井刀片分离该膜。传送含所述组织到一个孔含有1×PBS中的膜。
- 切割并用干净的解剖刀除去组织模型的侧面。然后切片的组织模型到4大致相等的方块。转移了一块组织到的Superfrost载玻片。存储所述组织片段中的1×PBS在4℃下。
- 干燥该组织5分钟,使用的ProLong Gold抗淬灭用DAPI和盖玻片安装。离开滑动,而不会干扰在黑暗中在室温至干。
注意:所述组织的厚度可以在中心和边缘之间变化,造成轻微tiltiNG盖玻片。为了避免这种情况,间隔(例如封口膜)可被放置在盖玻片的角落。 - 适用指甲油盖玻片的边缘,并且允许它干。沉浸在70%乙醇的幻灯片,使他们安全地衬托出BSL-3设施。
8,可视化,采集和定量三维分析
- 可视化使用共焦显微镜系统中的组织切片用激光以分别发射在488nm的GFP(绿色通道),420纳米为DAPI(蓝色)和555nm处的PKH26标记的单核细胞(红色)的激励。
- 1.5微米堆之间的分离 - 在带Z堆叠覆盖至少20微米厚,并具有1 512×512的分辨率获得的3D图像。 5-10获取不同领域覆盖了整片组织。
注:使用配置,如Nyqvist用于光学分辨率(波长,激光功率/曝光,像素大小和缩放)最佳设置。避免联合国明镜或以上的像素的饱和度。 - 分析共焦图像与三维图像处理软件。为细胞团的三维量化,被推荐为最佳分析以下步骤。
- 开启3D图像处理软件并加载图像。测量物体的图像中的待分析的尺寸,例如,一个核,单核细胞个体和单个细菌的大小。这些意见定义或过滤的对象很有用。
- 利用显示调节工具,通过调整各信道图像的对比度,亮度和混合的不透明度优化体积渲染。这一步骤是为了降低噪声的在体绘制的干扰。
注意:伽马校正可能导致操纵图像的,因此应该避免。 - 创建通过选择红色通道(单核细胞)的表面,并设置阈值(自动或手动选择)。如果需要,使用过滤器来限制红色单核细胞的选择或排除对Background。类似地,创建为绿色( 结核分枝杆菌 )和蓝(核)信道作为上述表面。
- 导出数据到MS-Excel文件。有可能导出特定参数或所有数据。有关的细胞的聚类分析的参数是体积,强度,对象数量,体素和球的数量。
- 保存并在一个合适的图像格式的TIFF优选导出图像。
注意:动画也可以用动画菜单制作并保存为一个媒体文件。 - 保存每个通道的使用Add参数选项的分析设置,稍后再使用重建功能可以检索。同时分析使用批量处理工具的详细文件。
注:所有待比较必须获取,处理并以相同的方式分析图像。例如,一个8位图像(256整数)不应与一个12位图像(4096整数)进行比较。
结果
甲三维肺组织模型为人类结核病可以有效地用于研究M中的宿主-病原体相互作用结核感染 。该方法的基本步骤,不同的步骤和组织切片的整体微观结构代表显微镜图像在图1中进行了概述。该模型具有人肺组织的几个部分组成,其中包括肺成纤维细胞,支气管上皮细胞和原代单核细胞/巨噬细胞嵌在三维组织的环境。除了结合人体肺组织的组成部分,该模型类似于生理条件,即分层上皮细胞和粘液分泌。
对于使用在监测结核感染的肺组织模型的一个例子示于图2中 ,对于可视化M.肺结核 -immune细胞迁移和互动,我们推出了感染M.巨噬细胞表达GFP 肺结核(绿色)连同新鲜分离PKH26标记的单核细胞(红色)进入组织模型(蓝色,DAPI染色的细胞核)。第7天,后加入M的结核病感染的细胞到组织模型中,共聚焦显微镜揭示了在感染(绿色)的部位(图2),它模仿人类结核病9的标志病变红色单核细胞簇。
一系列的代表图像为M的三维可视化结核病感染的组织模型和细胞簇的量化如图3的3D可视化使用户灵活地相互作用,检查和量化在3D图像的几个特点。绿菌和红色单核细胞簇的空间布置可从心尖,旋转和侧视图被看作如图 3B所示,它揭示了单核细胞的聚集在M的站点肺结核的集群 没有OB未感染的组织( 图3A)担任。我们量化单核细胞簇的大小和数量,发现细胞簇的大小(体积)的增强(P <0.001),而个别的单核细胞的数目M.降低 (p <0.01)相比于未感染的组织模型(图3C 和3D) 结核病感染的组织。这些数据验证了我们之前的早期肉芽肿形成的M.发现在2D组织切片9分析肺组织模型结核感染观察。
我们的数据显示,该组织模型提供了一个自然的3D栖息地调查复杂的宿主细胞- M.结核病的通信网络。我们还发现,三维可视化和定量分析是用于研究的特征在组织模型 (图3)更好的工具。小区集群的定量(肉芽肿例如)常常伸缩 HES几个细胞层,并且可以通过一个三维定量分析完全捕获。此外,单个细胞或细菌模型的准确时间和空间可视化功能允许在指定的实验室进行实时成像,迁移和跟踪研究。
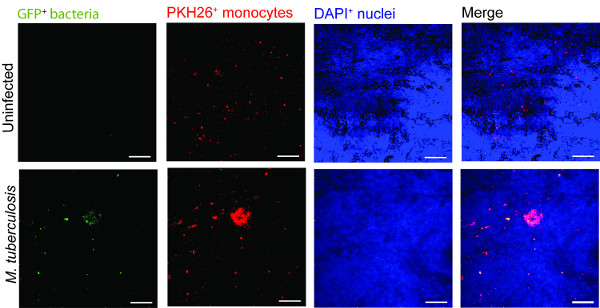
图2.单核细胞周围剧毒M.组织模型集群肺结核,未感染和 M.代表共聚焦图像结核感染的组织模型。板从绿色( 分枝的结核GFP),红色(PKH26标记的单核细胞),蓝色(DAPI染色细胞核)和合并的信道显示在感染组织的单核细胞的募集相比未感染的组织。规模 - 100微米。large.jpg"的风格="字体大小:14px的;的line-height:28px;"目标="_空白">点击此处查看该图的放大版本。
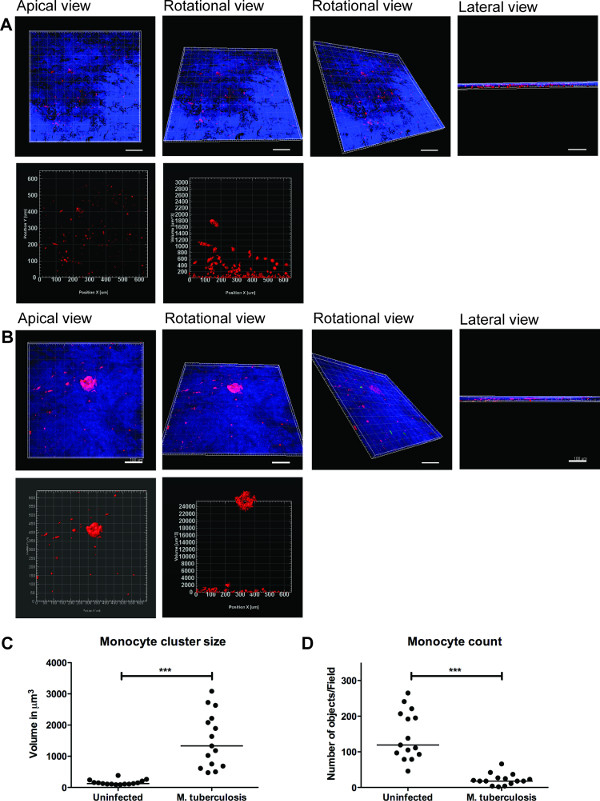
图3.三维可视化和组织模型的定量分析提供有用的信息。感染分枝整个组织模型(A)的未感染的组织,(B)的 3D可视化的代表性图像结核,通过使用蔡司LSM700共聚焦显微镜和定量分析了Imaris图像处理软件(7.6.8版本)光学切片。在20X放大率下获得这些图像,14的z堆叠覆盖的19.5微米的组织厚度1.5微米的间隔,允许从顶端,旋转水平,垂直旋转和横向视图(A和B)的可视化。(C)的第四纪分枝后早期肉芽肿单核细胞群的细胞团的ntitative分析揭示增加(P <0.0001)大小时相比,没有感染的结核病感染。(D)的单核细胞的数量的定量表明在感染组织的下降(P <0.01)相比,未感染的组织,重申多个聚类中的感染组织。绿色- M.结核病 - GFP,红色- PKH26标记的单核细胞,蓝-细胞核,尺度- 100微米请点击此处查看该图的放大版本。
讨论
The ability to recruit and form organized cell clusters at the site of infection is the hallmark of human TB 11. These dynamic structures known as tubercle granulomas primarily consist of immune cells (macrophages, monocytes, T-cells and B-cells) and multi-nucleated giant cells surrounding M. tuberculosis. The role of the granuloma has long been considered to wall off the infection, preventing local spread of bacteria. However, more recent studies show that granuloma formation is critical for early bacterial survival, growth and dissemination 12. A strategy of new studies is to identify molecules or pathways that could efficiently be targeted to inhibit the cellular migration in granuloma formation and/or TB dissemination.
A caveat for novel studies on TB is the lack of models that recapitulate human TB. The most widely used experimental animals do not form true granuloma upon M. tuberculosis infection, and are therefore not appropriate choices for studies of TB 13-16. Non-human primates have the closest resemblance to human TB 17, but are not the preferred choice owing to high operational costs and ethical issues. Human TB is a complex immunological process and is difficult to model in vitro. Cell cultures of monolayers or co-cultures lack the 3D environment and tissue responses. Therefore, we have developed an innovative lung tissue model based on human primary immune cells and human lung-specific cell lines 8,9. The model displays characteristic features of human lung tissue, including epithelia with evenly integrated macrophages, formation of extracellular matrix, stratified epithelia and mucus secretion 9.
The 3D human lung tissue model has several benefits over the in vitro single or co-cultures seeded on tissue culture plates or transwell inserts. First, the human lung-specific cells (fibroblasts and epithelial cells) are not commonly included in the in vitro single or co-cultures. Second, the immune cells and lung-specific cells are embedded in a 3D physiological context (collagen rich extra-cellular matrix products). The response of cells to a stimulus/infection and the migratory behaviour of cells, for instance formation of a granuloma, differ significantly between a 2D and 3D environment. Furthermore, the described method enables the 3D visualization and robust 3D quantitative analysis that provides pivotal information on spatial distribution and intricate cellular interactions.
Experimental infection in the model tissue with M. tuberculosis resulted in clustering of macrophages at the site of infection, reminiscent of early TB granuloma (Figure 2 and 3). We have recently demonstrated that mutant strains defective in the ability to secrete the virulence factor ESAT-6 or Mycobacterium bovis BCG that lacks ESAT-6 did not induce the clustering of monocytes (no early granuloma), in contrast to the virulent M. tuberculosis 9. These data are consistent with the observations made from Mycobacterium marinum-infected zebrafish embryos, whose transparency allows for elegant live imaging of granuloma formation 12. As there is no gold-standard model for TB, we took advantage of the surgically resected tissue biopsies from TB patients for validation of the method 9. Our in vitro tissue model shares several characteristics with the lung and lymph node biopsies from TB patients, including the aggregation of macrophages in granuloma, the presence of both intra- and extracellular bacteria 18 and induction of necrosis 11.
Although the described model has physiological relevance to human TB and has several advantages over other in vitro models, it has some limitations. For instance, out of more than 20 collagen proteins identified in humans, only type I is included to the model to mimic the extra-cellular matrix. However, type I collagen is a complex mixture of extra-cellular matrix products and is the most abundant collagen in the human body. Further, we have demonstrated the presence of collagen IV and several extra-cellular matrix proteins such as tropoelastin, vimentin and laminin, which are produced by the epithelial cells and fibroblasts in the tissue model, indicating the synthesis of new collagen 8. Presently, the lung tissue model only has monocytes and macrophages, besides lung-specific cells. It lacks neutrophils and lymphocytes that are also known to be present in the granuloma. Remarkably the model is not limited to the introduction of additional immune cells and is of interest to explore how they contribute to the complex cellular interactions in human TB. Implantation of primary alveolar macrophages, skin-specific cells and lung carcinoma cells has already been tested in the model. Since our objective was to use a model that closely resembles human TB, introduction of mouse cells have not been attempted.
In summary, the lung tissue model has implications for both basic mechanistic and applied studies. Potential applications of the lung model include the study of innate immunity, investigating mechanistic aspects of host defences such as phagosomal maturation, autophagy, production of cytokines, chemokines and anti-microbial peptides, and functional characterization of individual cell types. Strikingly, the in vitro tissue model allows manipulation of one or more cells types and provides a relevant tissue micro-environment, not only for studies on TB, but for a variety of infectious and non-infectious diseases that affect the lungs.
披露声明
The authors declare no competing financial interests.
致谢
The authors acknowledge the Microscopy core facility at the Faculty of Health Sciences, Linköping University for providing access to advanced imaging systems; Karl-Eric Magnusson (Emeritus Scientist) at the Dept. of Clinical and Experimental Medicine, Linköping University for providing access to Imaris 3D/4D image processing software (Bitplane, Switzerland); and S. Braian for his help with the lung model cartoon. This work was supported by funds from the Swedish Research Council (Alternatives to animal research, 2012-1951) and Swedish Research Council (2012-3349) to M.L. and Swedish Foundation for Strategic Research to S.B. S.B. receive grants from the Karolinska Institutet, Swedish Research Council, the Swedish International Development Cooperation Agency (Sida) and the Swedish Civil Contingencies Agency (MSB), and the Swedish Heart and Lung Foundation (HLF). M.S. received grants from the Karolinska Institutet and Stockholm County Council.
材料
| Name | Company | Catalog Number | Comments |
| Cell culture inserts | BD Falcon | 353092 | |
| 6-well culture plates | BD Falcon | 353046 | |
| MRC-5 cells, lung fibroblasts | ATCC#CCL-171 | ||
| 16HBE cells, lung epithelial cells | Gift from Dr. Dieter Gruenert, Mt. Zion Cancer Center, University of California, San Fransisco, USA | ||
| 5 x Dulbecco’s modified Eagle’s medium (5 x DMEM) | Gibco | 12800-082 | Made from powder but add 5 times less water. Adjust pH to 7.3 and filter it using a 0.2 µm filter. |
| Dulbecco’s modified Eagle’s medium with glucose (DMEM) 1x | Gibco | 41965-039 | |
| Minimum Essential Medium (MEM) 1x with Earle’s salts | Sigma | M4655 | |
| Non-Essential Amino Acids Solution, 100x | Life Technologies | 11140-035 | |
| L-glutamine 200 mM (100x) | Gibco | 25030-024 | |
| Sodium Pyruvate | Life Technologies | 11360-039 | |
| NaHCO3 (71.2 mg/ml) | Prepared in house | ||
| Heat inactivated Fetal Bovine Serum (FBS) | Gibco | 10270-106 | Heat inactivated for 30 min, 56 °C |
| Gentamicin (50 mg/ml) | Gibco | 15750-060 | |
| Hepes buffer solution 1M | Gibco | 15630-056 | |
| Penicillin Streptomycin (Pen Strep) | Gibco | 15140-122 | |
| Lymphoprep | Axis-Shield | 7801 | |
| Ultrapure 0.5 M EDTA | Gibco | 15575 | |
| Bovine Collagen PA treated (500 ml) | Organogenesis | 200-055 | |
| Pure col purified Bovine Collagen solution (100 ml) | Advanced biomatrix | 5005-B | |
| Extracellular matrix protein, Fibronectin (1 mg) | BD | 354008 | |
| Primary human monocytes/macrophages | Isolated from human whole blood or buffy coats. | ||
| PKH26 Red fluorescent cell linker | Sigma | MINI26 | |
| Mycobacterium tuberculosis H37Rv expressing green fluorescent protein | M. tuberculosis H37Rv wild type was transformed with the pFPV2 plasmid constitutively expressing GFP. | ||
| Middlebrook 7H9 medium | Difco | 271310 | |
| BBL Middlebrook ADC Enrichment | BBL | 211887 | |
| Tween-80 | |||
| Glycerol | |||
| Kanamycin B sulfate (20 µg/ml) | Sigma | B5264 | |
| Prolong Gold anti=-fade reagent with DAPI | Invitrogen | P36935 | |
| Trypsin -EDTA | |||
| Bovine serum albumin | |||
| Paraformaldehyde | |||
| DAPI | |||
| LSM700 Confocal microscope | Zeiss | ||
| iMaris Scientific 3D/4D image processing software, version 7.6.8 | Bitplane AG |
参考文献
- Sakamoto, K. The pathology of Mycobacterium tuberculosis infection. Veterinary pathology. 49, 423-439 (2012).
- Saunders, B. M., Britton, W. J. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. 85, 103-111 (2007).
- Kaufmann, S. H. New issues in tuberculosis. Ann Rheum Dis. Ann Rheum Dis. 63, ii50-ii56 (2004).
- Morrison, J., Pai, M., Hopewell, P. C. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 8, 359-368 (2008).
- Barry 3rd, C. E., et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 7, 845-855 (2009).
- Puissegur, M. P., et al. An in vitro dual model of mycobacterial granulomas to investigate the molecular interactions between mycobacteria and human host cells. Cell Microbiol. 6, 423-433 (2004).
- Kapoor, N., et al. Human Granuloma In Vitro Model, for TB Dormancy and Resuscitation. PLoS One. 8, e53657 (2013).
- Nguyen Hoang, ., T, A., et al. Dendritic cell functional properties in a three-dimensional tissue model of human lung mucosa. Am J Physiol Lung Cell Mol Physiol. 302, L226-L237 (2012).
- Parasa, V. R., et al. Modeling Mycobacterium tuberculosis early granuloma formation in experimental human lung tissue. Dis Model Mech. 7, 281-288 (2014).
- Cozens, A. L., et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 10, 38-47 (1994).
- Brighenti, S., Andersson, J. Local immune responses in human tuberculosis: learning from the site of infection. J Infect Dis. 205, S316-S324 (2012).
- Davis, J. M., Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 136, 37-49 (2009).
- Gupta, U. D., Katoch, V. M. Animal models of tuberculosis. Tuberculosis (Edinb). 85, 277-293 (2005).
- Kashino, S. S., Napolitano, D. R., Skobe, Z., Campos-Neto, A. Guinea pig model of Mycobacterium tuberculosis latent/dormant infection. Microbes Infect. 10, 1469-1476 (2008).
- Singhal, A., et al. Experimental tuberculosis in the Wistar rat: a model for protective immunity and control of infection. PLoS One. 6, e18632 (2011).
- Subbian, S., et al. Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium tuberculosis in rabbit lungs. PLoS Pathog. 7, e1002262 (2011).
- Lin, P. L., et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 62, 340-350 (2010).
- Rahman, S., et al. Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory t cells in the granulomatous lesions. Am J Pathol. 174, 2211-2224 (2009).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。