Method Article
单探头的应用:根据环境条件质谱成像和单细胞分析
摘要
Here, we present protocols to perform both ambient mass spectrometry imaging (MSI) of tissues and in-situ live single cell MS (SCMS) analysis using the single-probe, which is a miniaturized multifunctional device for MS analysis.
摘要
Mass spectrometry imaging (MSI) and in-situ single cell mass spectrometry (SCMS) analysis under ambient conditions are two emerging fields with great potential for the detailed mass spectrometry (MS) analysis of biomolecules from biological samples. The single-probe, a miniaturized device with integrated sampling and ionization capabilities, is capable of performing both ambient MSI and in-situ SCMS analysis. For ambient MSI, the single-probe uses surface micro-extraction to continually conduct MS analysis of the sample, and this technique allows the creation of MS images with high spatial resolution (8.5 µm) from biological samples such as mouse brain and kidney sections. Ambient MSI has the advantage that little to no sample preparation is needed before the analysis, which reduces the amount of potential artifacts present in data acquisition and allows a more representative analysis of the sample to be acquired. For in-situ SCMS, the single-probe tip can be directly inserted into live eukaryotic cells such as HeLa cells, due to the small sampling tip size (< 10 µm), and this technique is capable of detecting a wide range of metabolites inside individual cells at near real-time. SCMS enables a greater sensitivity and accuracy of chemical information to be acquired at the single cell level, which could improve our understanding of biological processes at a more fundamental level than previously possible. The single-probe device can be potentially coupled with a variety of mass spectrometers for broad ranges of MSI and SCMS studies.
引言
Mass spectrometry imaging (MSI) is a relatively new molecular imaging technique to provide the spatial distribution of the compounds of interest on surfaces. During the MSI analysis, mass spectrometry (MS) measurements are recorded across the surface on an individual pixel basis to create a 2D image of the species of interest 1. MSI techniques have the ability to provide a spatially resolved feature distribution for a large range of metabolites, allowing a much greater amount of information to be obtained from a sample than from using traditional molecular imaging techniques, and they have the potential to greatly improve the analysis of biological samples for biological and pharmacology studies 2. MSI can be broadly separated into non-ambient and ambient approaches. The non-ambient MSI analysis techniques, such as matrix assisted laser desorption ionization (MALDI) MS 3 and time of flight secondary ion MS (ToF SIMS) 4, are capable of high spatial resolution (around 5 µm and 100 nm, respectively) and high sensitivity. However, these methods require extensive sample preparation, such as the application of matrix molecules to the sample surface, and a vacuum sampling environment, which could introduce artifacts to the data obtained. Ambient techniques such as desorption electrospray ionization (DESI) MS 5, laser ablation electrospray ionization (LAESI) MS 6, and nano-DESI MS 7 are capable of MSI of samples with little to no prior preparation under the ambient environment, which is able to produce MS images that potentially reflect the sample in its most native state. However, most of these techniques generally lack the high spatial resolution and detection sensitivity compared with the non-ambient techniques, with experiments typically conducted at around 150 µm per pixel 8.
Single cell analysis (SCA) is a growing field that has the ability to characterize the chemical composition of biological samples at the cellular level. SCA enables the analysis of biological systems at a more fundamental level than traditional cell analysis techniques, which produce an averaged result of a population of cells, potentially providing insights that are previously intractable 9. MS techniques have recently been applied to SCA (termed single cell mass spectrometry or SCMS) using non-ambient techniques such as MALDI MS 10 and ToF SIMS 11 in which cells are pretreated before analysis, and with ambient techniques such as LAESI MS 12 and direct extraction methods, such as live single-cell video-MS 13, 14, to analyze a wide variety of cell types such as egg, plant, and cancer. Ambient techniques have the advantage of being applied to live cells, which again minimizes the artifacts, leading to a better representation of the metabolites in the live cells. The direct extraction based methods described above, however, perform the sample extraction and analysis process at two different steps, which result in a time gap during the analysis that could potentially alter the metabolites present within the sample.
The single-probe, a miniaturized multifunctional device that is capable of conducting high spatial resolution ambient MSI on biological tissue sections 15 and near real-time in-situ SCMS on live single cells 16. The single-probe has an integrated construction that is made up of a pulled dual-bore quartz capillary coupled with a solvent providing inlet and a nano-ESI emitter made from fused silica capillaries, enabling solvent delivery and analyte extraction to be performed from a single device. In the ambient MSI mode, the single-probe is placed over the sample tissue and surface micro-extraction occurs, allowing a rastered MS image to be made at high spatial resolution. Particularly, the tapered tip of the single-probe is small enough to be inserted into live eukaryotic cells for in-situ SCMS analysis, where the metabolite detection takes less than two seconds between probe insertion and MS detection, allowing chemical information to be taken in near real-time. Here are the protocols to fabricate the single-probe device and to conduct both the ambient MSI and SCMS modes using the single-probe MS techniques.
研究方案
动物使用和福利应坚持NIH指南实验动物以下的机构动物护理和使用委员会(IACUC)审查和批准协议的维护和使用。小鼠组织样品通过协作者Chuanbin毛博士提供。
1.鼠标组织切片准备
- 放置的兴趣(脑,肾,肝等 )成的一个小的塑料的中心的整个小鼠器官井( 例如 ,12孔细胞培养板),并浸没在组织包埋化合物高达约10毫米的高度。确保没有形成在组织包埋化合物并且该器官被放置在期望的方向( 即 ,矢状面,冠状等 )泡沫。
- 立即就地组织成闪光冷冻液态氮。对于长期储存,储存在-80℃冰箱中冷冻样品。
- 就拿冷冻鼠标器官和解冻至-15℃的temperatu重新控制冷冻切片。
- 安全组织到一个钢基底用约500微升组织灌封化合物的并放置到冷冻切片机切片装入使所需切片取向呈现给刀。
- 部的组织到12微米厚。放置切片组织切片到聚碳酸酯显微镜载玻片和离开干燥,在室温下30分钟。对于长期储存,储存在-80℃冰箱中冷冻滑动。
2.细胞培养
注:在无菌条件下在生物安全柜(生物安全Ⅱ级)进行细胞培养。 HeLa细胞被用作模型系统,以及细胞与下列常规协议完全培养基中培养:
- 温暖的试剂( 即 ,胰蛋白酶,磷酸缓冲盐水(PBS),和细胞培养基)至37℃。
注:细胞培养基包含无机山LTS,氨基酸,维生素及其他。对于成分的完整列表,请参阅从制造商的制定。 - 获得细胞样品( 例如 ,将1ml HeLa细胞悬浮液),并将其添加成9个ml完全细胞培养基中标准的10厘米细胞培养板。初始细胞数约为0.5×10 6个细胞/ ml。保持培养中的细胞在37℃,5%CO 2的2-3天,直到成长表面在70-80%覆盖在细胞培养板。对于每个连续的一轮创纪录的细胞传代次数。
- 执行在细胞培养板的细胞传代( 即 ,细胞分裂)。
- 吸出生长培养基,并使用5毫升1×PBS中冲洗细胞。除去PBS中使用无菌抽吸尖,孵育所述细胞在37℃C降低2.5 ml的胰蛋白酶(0.25%)〜5分钟,以从培养板分离细胞。
注意:实际胰蛋白酶处理时间需要根据具体胰蛋白酶机生产线来优化克拉从制造商购买。不足处理时间留下附着于板上的细胞,而过度的治疗导致细胞死亡。 - 通过加入7.5毫升完整细胞培养基停止胰蛋白酶活性,然后均匀地重悬细胞(总体积10ml)中。用文化(步骤2.2)或准备SCMS样品(步骤2.4)的细胞悬液。
- 吸出生长培养基,并使用5毫升1×PBS中冲洗细胞。除去PBS中使用无菌抽吸尖,孵育所述细胞在37℃C降低2.5 ml的胰蛋白酶(0.25%)〜5分钟,以从培养板分离细胞。
- 准备SCMS实验细胞样本。
- 放置单个微盖玻片在12孔平板,并添加1.8毫升细胞培养基和0.2 ml的细胞悬液的入井。
- 轻轻地用板的温和搅拌混合细胞,并在37℃下在5%的CO 2环境下孵育〜24小时。执行药物治疗来培养的细胞中,添加的药物化合物溶液( 例如 ,在DMSO(二甲基亚砜))到12孔细胞培养板。
注意:最终药物浓度( 例如 ,10纳米,100纳米,1微米和10微米)和叔reatment时间( 例如 ,4小时)根据研究的特定目的而变化。细胞附着到微盖滑动并准备好CSMS实验(步骤6)。
3.单探头制造
- 放置双孔的石英管(内径(ID)127微米,外径(OD)500微米)到一个激光微量拉马和拉双孔石英针。使用以下参数为出发点:热火= 400,费尔= 3,威赛= 80,德尔= 150,和普勒= 250(所有单位都是生产商的单位)。确保拉双口径石英针具有锥形尖探头的最佳性能。切断拉尖使得存在约5毫米长,另一端左unpulled双孔石英毛细管。
注意:激光拉出器的实际参数应根据仪器的具体条件进行优化。 - 切割熔融石英毛细管的〜80毫米a(ID 40微米,OD。 105微米)作为溶剂,提供毛细管,并且在双孔石英针的扁平端将其插入到一个孔。
- 切断熔融石英毛细管(ID 40微米,外径105微米)的一个〜40毫米节并用剃刀从中点剃〜5mm处的聚酰亚胺涂层。使用丙烷火焰快速加热和拉融合毛细管与细锥形纳米电喷雾离子化(ESI)发射器。切的纳米ESI发射器(〜7-10毫米长),并在双孔石英针的扁平端将其插入到其他孔。另外,使用激光车夫产生细锥形。
- 申请(〜1-2微升)的UV固化树脂的最小量到双孔石英针的平端,并用LED紫外线灯为〜20秒,以确保溶剂提供毛细管和纳米固化树脂ESI发射器。组装各个部件成一个单一的探头的程序示于图1a。
- 切一个标准显微镜GL驴滑动(1"×3")成半纵向。单探测器放置到载玻片使得纳米ESI发射极向外指向的一端。应用常规环氧树脂的单探头的主体,使得它成为固定在玻璃载片( 图1b)。留下过夜硬化。夫妇所制造的单探头与集成的单探头设置( 图1c),如在图1d中所示被附连到质谱仪。
4.构建集成的单探头MS安装
- 修改质谱仪的离子源接口凸缘和制造支架(可调节位置和高度)数字立体镜的( 图1E和1F)。
- 钻一个离子源接口凸缘与两个孔允许铝光学板的附着。做一个滑轨装置和高度调节杆(附用于精细位置调一XY工作台),使得所述数字立体镜系统可以连接到铝光板( 图1E)。
- 附加修改的数字立体显微镜,一个USB数字显微镜,具有柔性夹持器的微型手册XYZ平移台,电动XYZ平移台系统到铝光板,它安装在所述质谱仪的定制离子源接口凸缘( 图1c和图 1F)。使用柔性夹持器固定安装有单探头载玻片。
- 安装单探头设置到质谱仪( 图1F)。调整柔性夹持器和微型XYZ台放置单探头的发射极质谱仪中的入口的前方。上使用单探头的侧面的USB数字显微镜(可调视角),以提供单p的放大的图像单探针以上长袍尖端或纳米ESI发射极,并且所述数字立体镜(可调高度),以查看所述细胞和探头尖端。
注意:使用相应的离子源凸缘,此集成的单探头系统可耦合到任何其它类型的装备有环境电离源质谱仪。
5.环境MSI
- 解冻在室温下将样品部分,并将其放置到机动XYZ平移台系统的单探头下方。通过在控制软件改变的坐标调整样本位置。
- 使用注射器以适当的速率( 例如 ,0.2微升/分钟)泵送采样溶剂,并应用电离电压( 例如 ,5千伏)。采样溶剂的选择是灵活的,并且常见的包括甲醇:水(9:1)和乙腈。纳米ESI发射器的死体积估计为〜3 NL,及探针surfa之间的时间CE接触和离子信号观察通常小于1秒15。
注意:定制的离子源接口凸缘允许电离电压从质谱仪通过鳄鱼夹被递送到导电结合。电离电压,然后通过导电结合传递到毛细管和单探头通道内的溶剂中,并涂敷在纳米ESI发射电离取样分析物。确保连接用导电结合的鳄鱼夹,当电离电压被关断。 - 调整单探头,使其静息只是样品的表面上方并能够执行的代谢物的表面提取的高度。小心地提起的Z阶段,然后使用USB数字显微镜(在单探头侧)来监控单探头尖端和组织表面之间的距离的变化。该高度调节过程中监测该质谱的变化,并停止升降机ING的Z阶段是观察到的离子信号从溶剂背景下组织的代谢物的变化时。
- 重复步骤5.3三次以自动化表面平坦化调整阶段控制程序内设置三个不同的点。放置单探针在样品表面上三个点的尖端以彼此间隔开约10毫米的距离。按向上和向下的图标进行高度调节,以及三个点锁定到位"规划法"之下。
- 使用此程序样本内整个感兴趣的部分光栅其他参数进行设置。对于这里介绍的小鼠肾脏切片,用10.0微米/秒的速度光栅扫描和线之间20微米的距离。电动舞台系统具有0.1微米的最小增量运动。从步骤5.3中得到的单探针和组织之间的距离。
- 设置为MS谱的从质谱仪自动采集的方法。佛R ON小鼠肾脏样本高质量分辨率微星,使用下列参数:质量分辨率60,000(M /ΔM)〜5千伏的积极模式,1迈思肯,150毫秒的最大注入时间和AGC上。表示该MS图象的各条线都获得MS谱有相同数目的与每次扫描之间的均匀的时间间隔扫描,这表明对产生的图像的像素大小被均匀地分布。
- 启动微星的数据采集。发起用于质谱仪的MS采集序列,然后开始为XYZ控制程序的光栅扫描顺序。
- 例如,在这里使用的MS数据采集程序,转到"顺序设置",选择"新序列",产生一组文件从编号为01至X,其中X是用于该行数的新序列所需的MS图像进行拍摄,然后按"运行程序"。
- 使用自制的电子设备允许软件产生CONTA用于质谱仪克拉闭合信号以收集数据。的电路图中示出的补充图( 图S1)作为参考。
- 从构造采用适当的MSI可视化软件的原始MS文件MS图像。例如,使用由Laskin的组在PNNL 17开发的软件包时,执行以下步骤。
- 点击"眉毛文件"。选择从MSI实验中获得的第一个文件。指定下的文件开始和结束"行数"。下"输入MZ范围"中选择一个范围为MS图像范围m / z值的。
- 按"开始"按钮来启动映像创建过程。一旦MS图像时,点击"保存图像","工具栏"下的存储在计算机上的图像。
6. 在现场直播SCMS
- 设置单探头系统根据诲离子微星。调整溶剂( 例如,甲醇/ H 2 O或乙腈)流速( 例如 ,〜25标升/分钟)。
- 洗培养的细胞,其附着于微盖玻璃载玻片,用PBS以除去文化介质和胞外药物组分。将含有载玻片上的实验电动XYZ移动平台系统细胞。
注意:可替换地,可以使用新鲜的细胞培养液(不含胎牛血清)冲洗培养细胞。少离子抑制已被观察到。此外,细胞可以在实验在环境温度(〜20℃)比培养温度(37℃)显著降低期间较长时间存活。药物类型,溶液的浓度,处理时间在不同的研究有所不同。 - 聚焦数字立体显微镜(上面的样品)到单探头的尖端的分析期间监测细胞穿透。使用USB数字显微镜(在S单探针IDE)来监视单探头纳米ESI发射器的工作条件。
- 使用电动XYZ载物台控制程序和数字立体显微镜(上述细胞)来定位感兴趣的细胞,并精确地定位在样品上方的单一探针尖端。启动MS数据采集单探头尖端插入到电池之前。
- 质量分辨率100,000(米/ΔM),〜3千伏的正和负模式,1的Microscan,150毫秒的最大注射时间,在AGC模式:作为使用高分辨质谱仪MS分析引用使用以下参数。自动MS光谱的获取是通过单击MS数据采集程序"开始"完成。
- 通过点击穿透细胞膜,并保持记录从细胞生成的MS信号的图标提起电动Z-工段。 1-2秒的时间延迟,通常是在探针插入和MS信号检测之间观察到。作为其他的确认细胞穿透,该MS的信号的急剧变化可以根据细胞膜的渗透被观察到。细胞内的化合物的MS信号通常可以持续〜前15-20秒显著下降。
- 下部向下含板从细胞拉单探针尖端出细胞。它通常需要<15秒为蜂窝化合物的离子信号,以接近噪声电平。让溶剂流~3分钟后,完全刷新单探头。同时,定位在机动XYZ载物台系统,以定位下一个细胞进行分析。每个小区实验需要〜3分钟以完成。
结果
单探针成功地用于切片小鼠肾组织15的周围的MSI分析。该装置采用表面液体微萃取( 图1a),它提供了从一个小区域高效的分析物的提取,从而导致在对MSI结果丰富离子信号强度的机制。例如,超过10 7的信号强度已经达到了某些丰富代谢物( 图2a)。以这种方式检测到大量的代谢物,包括一些鞘磷脂(SM)和磷脂酰胆碱(PC)的物种如[SM(34:1)+钠] +(725.5575米/ Z),[PC(32: 0)+ H] +(734.5700米/ Z),[PC(34:1)+钠] +(782.5696米/ Z),和[PC(38:5 + NA)] +(814.5726米/ z)表示 。这些化合物被认定具有较高的质量分辨率和质量准确度加上t表示OA高分辨质谱仪。例如,该识别用小于4ppm的M / Z的质量精确度( 即 ,所观察到的和理论值之间的差异)为每代谢物( 图2b)在结果中这里提出实现。此外,串联质谱分析( 即 ,MS / MS)也被用于感兴趣物种的更自信鉴定进行。15
由于在小区域进行高效液体微萃取的能力,单探针装置可用于环境条件15下进行高空间分辨率的MSI实验。例如,已经获得示出所选代谢物( 图2c)的空间分布小鼠肾切片的详细的MS的图像。该MS图像的空间分辨率被确定为8.5微米,以下具有transiti的广泛使用的度量。在点MS信号的20-80%的强度变化而确定的一个尖锐特征的18磷脂的情况下,[PC(38:5 + NA)] +鼠标肾脏部分,内髓质之间的转换功能和外髓质发生在记时穿过一个扫描周期的地方,呈现出强度变化大于20-80%的范围内。基于样品移动速度(10.0微米/秒)和MS数据采集速率(0.85秒/光谱),将样品移动在一个MS距离扫描周期(8.5微米), 即 ,对MSI的空间分辨率,就可以计算( 图2D)。这种空间分辨率是当中最高的为生物样品进行环境MSI技术尚未实现。
对于SCMS单探头能够实现单个活HeLa细胞16的分析。单探针的尖端尺寸通常小于10微米( 图URE 3a)中,这是足够小,可以直接插入到多种类型真核细胞中的其直径为〜10微米,用于提取和MS分析。单探针针尖到细胞的插入过程可使用数字立体显微镜在视觉上监视( 图3b),和细胞膜穿透可以通过从PBS(或新鲜的细胞培养质谱的快速和显著变化来确认培养基)于细胞内的化合物( 图3c和3d)。该实验可以在正和负离子模式下进行,以检测更广种类的分子种。例如,18个不同的脂质种类在正模式进行了鉴定,包括鞘磷脂(SM)和磷脂酰胆碱(PC),而磷酸腺苷(AMP,ADP和ATP)在负离子模式( 图3c和d)检测。单探针插入我之间的时间延迟n要一个小区和所述信号检测是通常不到两秒钟,使细胞代谢产物的近实时检测。 SCMS还应用于其中细胞用抗癌药( 例如 ,OSW-1,紫杉醇,多柔比星)19]治疗实验。相应的药物可HeLa细胞内后4小时的治疗在一系列浓度( 即 10纳米,100纳米,1微米和10微米)的DMSO(二甲基亚砜),使用未处理的细胞(仅添加DMSO来检测)作为对照。药物的MS信号不是胞外PBS或对照( 图3e)内本,但是使用单探头MS技术(仅100nM的治疗结果示于图3f)的单电池内进行检测。因为细胞用PBS(或新鲜细胞培养基)漂洗,以除去细胞外的化合物和污染物,内源性代谢物( 例如 ,细胞的脂质一个的检测ð磷酸腺苷)和外源性化合物( 例如 ,抗癌药物),表示该单探测器MS技术可用于分析细胞内的化合物。
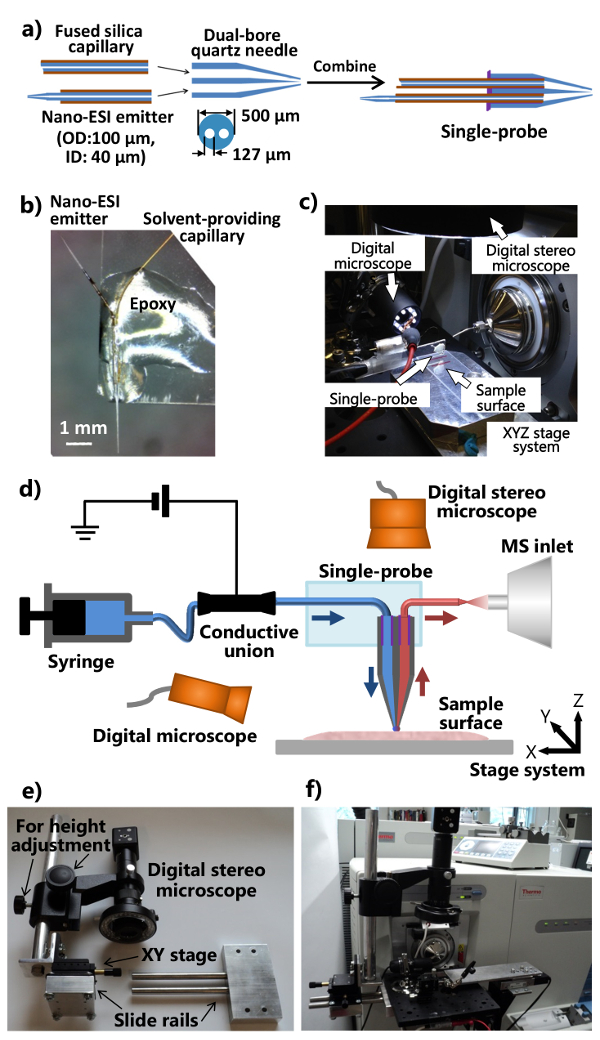
图1.制备和单探针用于环境MSI和SCMS分析的设置。 一个 )单探针的制作过程。B)附接至载玻片。c)该单的照片一制造的单探头的照片附着到质谱仪。D探测器设置)加上质谱仪单探头设置的示意图。在实验过程中,连续地从注射器中提供的采样溶剂,电离电压从质谱仪施加到导电结合,两种数字显微镜被用来监测样品的位置,电动XYZ台系统是用来控制样品的运动,以及一个质谱仪用于分析。e)该定制数字立体镜系统。 六 )照片显示通过光学板附着到离子源接口凸缘数字立体镜的照片。 请按此查看该图的放大版本。
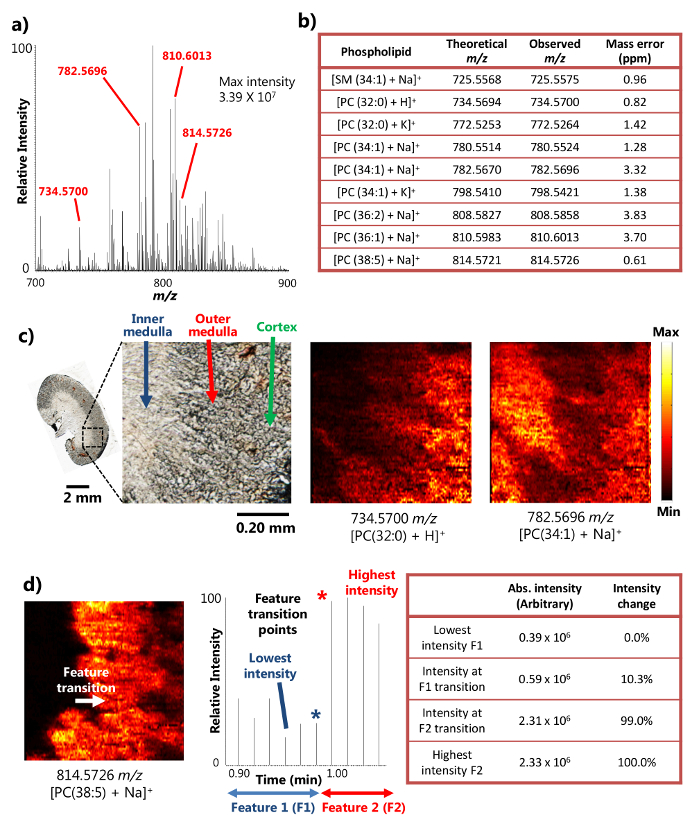
图2.从高空间和质量分辨率的小鼠肾脏部的周围的MSI研究的结果。a)由单探测器的MSI的代表性质谱。检测到的代谢物能达到3.39×10 7(任意单位)的最大强度。B)检测到的代谢物的选择提出了他们的质量精确度。C)MS的图像[PC(32:0)+ H] +和[PC(34:1)+钠] +在8.5微米的空间分辨率从小鼠肾脏部分采取。 PC:磷脂酰胆碱。比例尺:2毫米; 0.20毫米(插图)的MS图像的空间分辨率d)确定。[38 PC(5)+钠] +(改编许可,从参考15)。 请点击此处查看该图的放大版本。

图3.药物治疗的HeLa细胞具有较高的质量分辨率的环境SCMS分析结果。 一 )放大后的显示直径<10微米的典型尺寸单探头端部的照片。b)照片拍摄于点单探头插入到HeLa细胞。比例尺:50微米。c)与一些PC(磷脂酰胆碱)的物种。 四 )从HeLa细胞均在正离子和负离子模式。EF)质谱用于SCMS分析检测出的代谢物的代表性例子的标识的典型正离子模式质谱控制与处理(100纳米OSW-1)细胞(改编许可,从参考16)。 请点击此处查看该图的放大版本。
图S1。用于生产触点闭合信号,质谱仪收集数据的电子设备的电路图。 请点击此处查看或下载这个数字。
讨论
单探针是可同时用于MSI和SCMS实验的多功能设备。单探头设置(包括平移台系统,显微镜,离子源接口凸缘等 )被设计为可灵活地适应于现有的质谱仪的附加 组件。单探头设置和常规ESI离子源之间的快速交换可以在一分钟内完成。原则上,使用适当的离子源接口凸缘,单探头设置可适应于任何其他质谱仪。此外,可以用单探头设置为反应性MSI和SCMS实验,这大大增强了生物分子的更广泛的范围内的检测可以使用含有各种试剂的采样溶剂。除了动物组织和细胞系,该单探测器还能够分析其他生物系统如植物。因此,用相同的实验装置和相似用户培训,可以使用单个仪器中进行的各种研究和用相同的用户,允许高效率和通用性实验将具有最小的训练时间和仪器费用实现。
单探针MS技术的关键组成部分是探针自身。单探头的质量对其性能,这在很大程度上决定了无论MSI和SCMS实验的质量有显著的影响。当制作单探头,确保双孔管内的毛细管牢固地粘到消除溶剂泄漏的实验过程中的机会。它是用紫外线可固化的环氧树脂的最小量,使得该孔和毛细管探针制造过程中不会堵塞的关键。
单探针已被用于在生物样品15进行高空间和质量分辨率环境的MSI。环境MSI的主要优势非环境的方法是,样品的制备是保持在最低,无需真空采样环境,其允许样品在近天然状态8进行分析。其中大多数其它环境MSI技术的主要障碍是缺乏空间分辨率1。与基于解吸相比微星技术(如DESI和LAESI),单探针的小尖尺寸允许小区域里进行的更健壮和有效的表面液体微萃取,导致一个高空间分辨率8.5微米,这是众多使用环境的MSI技术15达到的最高的。此外,调整采样溶剂的组分提供了额外的灵活性来进行实验。例如,抽样含有试剂( 例如 ,双阳离子化合物)的溶剂已被用于执行反应性的MSI的实验中,允许在代谢物确定PE的数目显著增加- [R实验20。单探针的另一优点是集成的设计,这在整个数据采集过程提供了操作方便。因为尖端和组织表面之间的距离为离子信号强度和稳定性,从而获得平的组织切片,并进行表面平坦化调整以最小化的距离方差非常敏感为高品质的MSI实验的关键。它遵循单探针微星技术不适合于获得不平整的表面的高空间MS的图像。
除了制造高品质的探头,仔细调整仪器为成功的MSI实验是必不可少的。在所有调整步骤,调整所述单探针尖端组织切片表面上方的高度是最关键的。当调整探头高度,泵采样溶剂并开启电离电压,使得只有溶剂背景离子信号可OBSERV编辑。然后监视质谱的变化,同时小心地通过提升机动Z台直到可以观察到从组织切片强和稳定的离子信号减少探针表面的距离;此探测器高度将在实验过程中被用于MSI数据集合。此外,优化的溶剂流速为MSI实验是必不可少的。调整用优化探针高度的流速。确保有组织表面上无溶剂扩散( 即 ,流率太高)或纳米ESI发射器( 即 ,流速太低)内气泡的形成。
单探针是用于生物分析的多功能设备。除了 对MSI的实验中,它是能够接近实时原位 SCMS阐明从活真核细胞16详细的化学信息,这是一个主要的优点与其他真空相比导电的基于SCMS技术(如MALDI 10和SIMS 21 )。探针尖端的小尺寸提供了被插入到活真核细胞和提取和电离立即MS分析细胞内的化合物的能力。同样地,可以在该SCMS实验中使用含有试剂( 例如 ,双阳离子化合物)的采样的溶剂,并且可以在比以往一个活的单细胞被检测细胞成分的更广泛的范围(正在进行的研究中,数据未显示)。虽然实时分析将提供活的单细胞由于膜和细胞内容物提取的细胞穿透的化学性质,所研究的细胞将在实验后被杀死,这意味着单探头SCMS技术仍是一个破坏性的方法。另外,探针和纳米ESI发射极中的单探针可以容易堵塞对于没有经验的用户。以降低装置堵塞的机会,确保避免插入单探头插入一个CEL当触摸核湖如果发生堵塞,该装置可以通过加热堵塞探头尖端或纳米ESI发射使用自制的加热线圈16进行再生。单探针SCMS技术的另一个限制是,只有粘合剂的细胞( 即 ,细胞附着于表面)可以用当前的设置进行分析。然而,通过将细胞操纵系统进入单探头MS装置,更广泛类型的细胞可以在将来的研究。
类似的MSI实验,获得高品质的探针和优化的溶剂流速为SCMS的研究是至关重要的。当调谐溶剂流速,单探针尖端放置在样品上方( 即 ,与细胞或培养基不接触),并保证有从探头尖端或纳米ESI内形成气泡无溶剂滴落发射器。
披露声明
We have no conflict of interest to declare with the work presented here.
致谢
The authors would like to thank Dr. Laskin (the Pacific Northwest National Laboratory) for sharing the motorized stage control software and MSI visualization program. We also thank Dr. Mao (the University of Oklahoma) for providing mouse organ samples and Mr. Chad E. Cunningham (the University of Oklahoma) for the assistance in machining and electronics work. This research was supported by grants from the Research Council of the University of Oklahoma Norman Campus, the American Society for Mass Spectrometry Research Award (sponsored by Waters Corporation), Oklahoma Center for the Advancement of Science and Technology (Grant HR 14-152), and National Institutes of Health (R01GM116116).
材料
| Name | Company | Catalog Number | Comments |
| Single-probe fabrication | |||
| Dual bore quartz tubing, 1.120’’ × 0.005” × 12” | Friedrich & Dimmock, Inc, Millville, NJ | MBT-005-020-2Q | |
| Micropipette laser puller | Sutter Instrument Co., Novato, CA | Model P-2000 | |
| Fused silica capillary, ID: 40 µm, OD: 110 µm | Molex, Lisle, IL | TSP040105 | |
| UV curing resin | Prime Dental, Prime-Dent, Chicago, IL, USA | Item No. 006.030 | |
| LED UV lamp | Foshan Liang Ya Dental Equipment, Guangdong, China | LY-C240 | |
| Epoxy resin | Devcon, Danvers, MA | Part No. 20945 | |
| Inline MicroFilter | IDEX Health & Science LLC, Lake Forest, IL | M-520 | |
| Microunion | IDEX Health & Science LLC, Lake Forest, IL | M-539 | |
| Microscope slide (glass) | C & A Scientific - Premiere, Manassas, VA | 9105 | |
| Syringe | Hamilton, Reno, NV | 1725LTN 250UL | |
| Mass spectrometer | |||
| LTQ Orbitrap Mass sprectrometer | Thermo Fisher Scientific, Inc., Waltham, MA | LTQ Orbitrap XL | |
| Xcalibur 2.1 Software | Thermo Fisher Scientific, Inc., Waltham, MA | XCALIBUR21 | |
| Fance Stage Control | Pacific Northwest National Laboratory, Richland, WA | ||
| MSI QuickView | Pacific Northwest National Laboratory, Richland, WA | ||
| Contact closure device | |||
| USB-6009 Multifunction DAQ | National Instruments, Austin, TX | 779026-01 | |
| DR-5V SDS Relay | Panasonic, Kadoma, Japan | DR-SDS-5 | |
| Logic Gates 50 Ohm Line Driver | Texas Instruments, Dallas, TX | SN74128N | |
| Single-probe setup | |||
| Motorized linear stage and controller (3 sets) | Newport, Irvine, CA | Conex-MFACC | |
| Miniature XYZ stage | Newport, Irvine, CA | MT-XYZ | |
| Translation XY stage | ThorLab, Newton, NJ | PT1 and PT102 | |
| Thermo LTQ XL ion source interface flange | New Objective, Woburn, MA | PV5500 | |
| Digital stereo microscope, 250X - 2,000X | Shenzhen D&F Co., Shenzhen, China | Supereyes T004 | |
| USB Digital Photography Microscope | DX.com, HongKong, China | S02 25~500X | |
| Syringe pump | Chemyx Inc., Stafford, TX | Nexus 3000 | |
| Solid Aluminum Optical Breadboard, 8" x 8" x 1/2" | Thorlabs, Newton, NJ | MB810 | |
| Flexible clamp holder | Siskiyou, Grants Pass, OR | MXB-3h | |
| Solvents | |||
| Methol | Sigma-Aldrich, St. Louis, MO | 34860 Chromasolv | |
| Water | Sigma-Aldrich, St. Louis, MO | W4502 | |
| Acetonitrile | Sigma-Aldrich, St. Louis, MO | 34967 Chromasolv | |
| Cell culture | |||
| Dulbecco’s Modified Eagle’s Medium (DMEM) | Cellgro, Manasas, VA | 10-013-CV | |
| 10% heat-inactivated fetal bovine serum (FBS) | Gibco/Life Technologies, Long Island, NY | 10100-139 | |
| Penicillin/Streptomycin | Cellgro, Manasas, VA | 30-002-CI | |
| 10 mM HEPES (pH 7.4) | Cellgro, Manasas, VA | 25-060-CI | |
| Phosphate Buffered Saline (PBS) | Cellgro, Manasas, VA | 46-013-CM | |
| TrypLE Express | Thermo Fisher Scientific, Waltham, MA | 12604-013 | |
| 12-well plates | Corning Inc., Corning, NY | Falcon 351143 | |
| T25 flask | Corning Inc., Corning, NY | Falcon 3055 | |
| Micro Cover Glasses, Round, No. 1 | VWR International, Radnor, PA | 48380-046 | |
| DMSO (Dimethyl Sulfoxide) | VWR International, Radnor, PA | BDH1115-1LP | |
| Tissue imaging | |||
| Cyro-Cut Microtome | American Optical Coporation | ||
| Tissue-Tek, Optimum cutting temperature (OCT) | Sakura Finetek Inc., Torrance, CA | 4583 | |
| Microscope slide (polycarbonate) | Science Supply Solutions, Elk Grove Village, IL | P11011P | |
参考文献
- Vickerman, J. C. Molecular imaging and depth profiling by mass spectrometry-SIMS, MALDI or DESI?. Analyst. 136 (11), 2199-2217 (2011).
- Schwamborn, K. Imaging mass spectrometry in biomarker discovery and validation. J. Proteomics. 75 (16), 4990-4998 (2012).
- Schwamborn, K., Caprioli, R. M. MALDI Imaging Mass Spectrometry - Painting Molecular Pictures. Mol Oncol. 4 (6), 529-538 (2010).
- Kraft, M. L., Klitzing, H. A. Imaging lipids with secondary ion mass spectrometry. Biochim. Biophys. Acta. 1841 (8), 1108-1119 (2014).
- Wiseman, J. M., et al. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proc Natl Acad Sci U S A. 105 (47), 18120-18125 (2008).
- Nemes, P., Woods, A. S., Vertes, A. Simultaneous imaging of small metabolites and lipids in rat brain tissues at atmospheric pressure by laser ablation electrospray ionization mass spectrometry. Anal. Chem. 82 (3), 982-988 (2010).
- Laskin, J., Heath, B. S., Roach, P. J., Cazares, L., Semmes, O. J. Tissue Imaging Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 84 (1), 141-148 (2012).
- Wu, C., Dill, A. L., Eberlin, L. S., Cooks, R. G., Ifa, D. R. Mass spectrometry imaging under ambient conditions. Mass. Spectrom. Rev. 32 (3), 218-243 (2013).
- Altschuler, S. J., Wu, L. F. Cellular heterogeneity: do differences make a difference?. Cell. 141 (4), 559-563 (2010).
- Amantonico, A., Urban, P. L., Fagerer, S. R., Balabin, R. M., Zenobi, R. Single-Cell MALDI-MS as an Analytical Tool for Studying Intrapopulation Metabolic Heterogeneity of Unicellular Organisms. Anal. Chem. 82 (17), 7394-7400 (2010).
- Passarelli, M. K., Ewing, A. G., Winograd, N. Single-cell lipidomics: characterizing and imaging lipids on the surface of individual Aplysia californica neurons with cluster secondary ion mass spectrometry. Anal. Chem. 85 (4), 2231-2238 (2013).
- Shrestha, B., et al. Subcellular metabolite and lipid analysis of Xenopus laevis eggs by LAESI mass spectrometry. PLoS One. 9 (12), e115173 (2014).
- Mizuno, H., Tsuyama, N., Harada, T., Masujima, T. Live single-cell video-mass spectrometry for cellular and subcellular molecular detection and cell classification. J. Mass. Spectrom. 43 (12), 1692-1700 (2008).
- Zhang, L., et al. In Situ metabolic analysis of single plant cells by capillary microsampling and electrospray ionization mass spectrometry with ion mobility separation. Analyst. 139 (20), 5079-5085 (2014).
- Rao, W., Pan, N., Yang, Z. High Resolution Tissue Imaging Using the Single-probe Mass Spectrometry under Ambient Conditions. J. Am. Soc. Mass. Spectrom. 26 (6), 986-993 (2015).
- Pan, N., et al. The single-probe: a miniaturized multifunctional device for single cell mass spectrometry analysis. Anal. Chem. 86 (19), 9376-9380 (2014).
- Thomas, M., et al. Visualization of High Resolution Spatial Mass Spectrometric Data during Acquisition. 2012 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc). , 5545-5548 (2012).
- Luxembourg, S. L., Mize, T. H., McDonnell, L. A., Heeren, R. M. High-spatial resolution mass spectrometric imaging of peptide and protein distributions on a surface. Anal. Chem. 76 (18), 5339-5344 (2004).
- Zhou, Y., et al. OSW-1: a natural compound with potent anticancer activity and a novel mechanism of action. J Natl Cancer Inst. 97 (23), 1781-1785 (2005).
- Rao, W., Pan, N., Tian, X., Yang, Z. High-Resolution Ambient MS Imaging of Negative Ions in Positive Ion Mode: Using Dicationic Reagents with the Single-Probe. J. Am. Soc. Mass. Spectrom. 27 (1), 124-134 (2016).
- Ostrowski, S. G., Kurczy, M. E., Roddy, T. P., Winograd, N., Ewing, A. G. Secondary ion MS imaging to relatively quantify cholesterol in the membranes of individual cells from differentially treated populations. Anal. Chem. 79 (10), 3554-3560 (2007).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。