Structure Of Ferrocene
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
In 1951, Kealy and Pauson reported to Nature the synthesis of a new organometallic compound, ferrocene.1 In their original report, Pauson suggested a structure for ferrocene in which the iron is singly bonded (sigma bonds) to one carbon atom of each cyclopentadiene ligand (Figure 1, Structure I).1,2,3 This initial report led to wide-spread interest in the structure of ferrocene, and many leading scientists participated in the structure elucidation of this interesting new molecule. Wilkinson and Woodward were quick to suggest an alternative formulization where the iron is "sandwiched" between two cyclopentadiene ligands, with equal binding to all 10 carbon atoms (Figure 1, Structure II).4 Here, we will synthesize ferrocene and decide, based on experimental data (IR and 1H NMR), which of these structures is observed. In addition, we will study the electrochemistry of ferrocene by collecting a cyclic voltammogram. In the course of this experiment, we introduce the 18-electron rule and discuss valence electron counting for transition metal complexes.
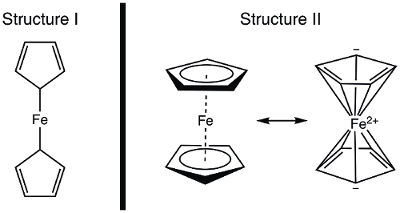
Figure 1. Two proposed structures of ferrocene.
1. Cracking the Cyclopentadiene Dimer (Figure 3)
Cyclopentadiene undergoes a Diels-Alder reaction with itself to give dicyclopentadiene. This reaction is reversible, so cracking is accomplished using La Châtelier's principle to drive the reverse reaction by distilling the cyclopentadiene monomer (b.p. 42 °C) away from the dicyclopentadiene dimer (b.p. 170 °C). The dimerization reaction is slow when the cyclopentadiene is kept cold, but it must be
Ferrocene Characterization:
1H NMR (chloroform-d, 300 MHz, δ, ppm): 4.15 (s).
The 1H NMR spectrum of ferrocene clearly shows a single resonance, consistent with structure II.
A CV of ferrocene is given below. The E1/2 value obtained for the oxidation of ferrocene was +90 mV (acetonitrile, scan rate 100 mV/s, 0.1 M (Bu4N)PF6, glassy carbon working electrode). The ferrocene/ferrocenium redox couple is commonly used as a reference in cyclic voltam
In this video, we discussed ferrocene and the role it played in the development of organometallic chemistry. Ferrocene was synthesized and characterized by 1H NMR and IR spectroscopy. Both spectra are consistent with the 18 e− Structure II, where the iron is "sandwiched" between two cyclopentadiene ligands, with equal binding to all 10 carbon atoms (Figure 1, Structure II). Oxidation of ferrocene to ferrocenium cation was observed electrochemically.
1. Kealy, T. J., Pauson, P. L. A New Type of Organo-Iron Compound. Nature. 168 (4285), 1039-1040 (1951).
2. Pauson, P. L. Ferrocene—how it all began. J Organomet Chem. 637, 3-6 (2001).
3. Seeman, J. I., Cantrill, S. Wrong but seminal. Nat Chem. 8 (3), 193-200 (2016).
4. Wilkinson, G., Rosenblum, M., Whiting, M. C., Woodward, R. B. The Structure of Iron Bis-cyclopentadienyl. 74, 2125-2126 (1952).
5. Green, M. L. H., Parkin, G. Application of the Covalent Bond Classification Method for the Teaching of Inorganic Chemistry. J Chem Educ. 91 (6), 807-816 (2014).
6. Press Release. http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1973/press.html.
7. Crabtree, R. H. The Organometallic Chemistry of the Transition Metals, 6th ed. John Wiley & Sons. Hoboken. 2014.
8. Gildner, P. G., Colacot, T. J. Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings. Organometallics. 34, 5497-5508 (2015).
关于 JoVE
版权所属 © 2024 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。