The DREAM Implant: A Lightweight, Modular, and Cost-Effective Implant System for Chronic Electrophysiology in Head-Fixed and Freely Behaving Mice
* These authors contributed equally
In This Article
Summary
Here, we introduce a lightweight, cost-effective probe implant system for chronic electrophysiology in rodents optimized for ease of use, probe recovery, experimental versatility, and compatibility with behavior.
Abstract
Chronic electrophysiological recordings in rodents have significantly improved our understanding of neuronal dynamics and their behavioral relevance. However, current methods for chronically implanting probes present steep trade-offs between cost, ease of use, size, adaptability, and long-term stability.
This protocol introduces a novel chronic probe implant system for mice called the DREAM (Dynamic, Recoverable, Economical, Adaptable, and Modular), designed to overcome the trade-offs associated with currently available options. The system provides a lightweight, modular and cost-effective solution with standardized hardware elements that can be combined and implanted in straightforward steps and explanted safely for recovery and multiple reuse of probes, significantly reducing experimental costs.
The DREAM implant system integrates three hardware modules: (1) a microdrive that can carry all standard silicon probes, allowing experimenters to adjust recording depth across a travel distance of up to 7 mm; (2) a three-dimensional (3D)-printable, open-source design for a wearable Faraday cage covered in copper mesh for electrical shielding, impact protection, and connector placement, and (3) a miniaturized head-fixation system for improved animal welfare and ease of use. The corresponding surgery protocol was optimized for speed (total duration: 2 h), probe safety, and animal welfare.
The implants had minimal impact on animals' behavioral repertoire, were easily applicable in freely moving and head-fixed contexts, and delivered clearly identifiable spike waveforms and healthy neuronal responses for weeks of post-implant data collection. Infections and other surgery complications were extremely rare.
As such, the DREAM implant system is a versatile, cost-effective solution for chronic electrophysiology in mice, enhancing animal well-being, and enabling more ethologically sound experiments. Its design simplifies experimental procedures across various research needs, increasing accessibility of chronic electrophysiology in rodents to a wide range of research labs.
Introduction
Electrophysiology with chronically implanted silicon probes has emerged as a powerful technique for investigating neural activity and connectivity in behaving animals, particularly in mice, due to their genetic and experimental tractability1. Laminar silicon probes, in particular, have proven to be an invaluable tool for identifying functional relationships within cortical columns2 and for relating the dynamics of large neuronal populations to behavior in a way that was impossible previously3.
Two complementary approaches are the current gold standards for recording neural activity in vivo: two-photon microscopy4,5 and extracellular electrophysiology6. The choice of recording methodology constrains the nature of the readouts that can be obtained: two-photon microscopy is particularly well-suited to longitudinal studies of individually identifiable neurons in large populations across time but suffers from high equipment costs and is limited to superficial layers of the cortex in intact brains. In addition, the typical temporal resolution of ~30 Hz limits its ability to capture ongoing neuronal dynamics7,8.
In contrast, electrophysiological recordings offer high temporal resolution (up to 40 kHz) to track neuronal activity moment by moment, can be applied widely across species as well as across cortical depths, and have relatively low-cost setups compared to two-photon microscopy. However, the identification of individual neurons, as well as longitudinal tracking of neuronal populations, are difficult to achieve. This especially applies to wire electrodes, e.g., tetrodes, and to acute electrode insertions. Besides lacking the ability to track neurons across recording sessions9, repeated acute insertions cause local trauma10 that mounts an immune response11, increasing the chance of infection and gliosis. This ultimately reduces the stability of recorded neuronal activity and life expectancy of experimental animals, limiting the scope of longitudinal studies featuring acute electrophysiological recordings to just a few days12.
Chronic high-density silicon probe recordings aim to combine some of the best attributes of acute electrophysiology and two-photon imaging. They can track neural population dynamics across sessions with only a somewhat lowered ability to identify individual neurons compared to two-photon imaging13. These recordings provide high flexibility in the spatial placement and precise temporal resolution of the recorded signals, as well as improved longevity and well-being of experimental animals compared to acute recordings14. Furthermore, in contrast to acute recordings, chronic electrophysiology necessitates only a single implantation event, effectively reducing the risk of infection and tissue damage and minimizing stress on the animals15. Collectively, these advantages make chronic electrophysiology a powerful tool for investigating the organization and function of the nervous system.
However, commonly used chronic implantation techniques for mice constrain researchers to make significant trade-offs between compatibility with behavioral recordings, implant weight, replicability of implants, financial costs, and overall ease of use. Many implant protocols are not designed to facilitate the reuse of probes16, steeply raising the effective cost of individual experiments and thus making it financially difficult for some labs to use chronic electrophysiology. They also often require extensive in-house prototyping and design work, for which the expertise and resources may not be present.
On the other hand, integrated implant systems17 offer a more widely accessible solution for chronic electrophysiology in rodents. These systems are designed to integrate a microdrive holding the probe with the remainder of the implant to simplify implant handling and surgical procedures. However, once implanted, such systems can be top-heavy and limit the experimenter's ability to flexibly adapt an experiment to different target coordinates. Often, their weight precludes implants in smaller animals, potentially impairs animal movement and induces stress18. This can disproportionately affect research on juvenile and female cohorts, as weight limitations are more likely to affect these groups.
Additionally, not all integrated systems allow for adjustment of electrode positions post-implantation. This is relevant, as gliosis or scarring due to probe insertion19, especially in the initial 48 h after implantation20, can reduce the quality of the recorded neuronal activity. Micro-adjustments to the probe insertion depth can limit these negative effects on signal integrity. Therefore, micropositioning mechanisms, commonly called microdrives, can be beneficial even in probes with a large number of electrodes distributed across their length.
To overcome such trade-offs, we introduce a novel chronic electrophysiology implant system for mice that addresses the limitations of previous designs by offering a lightweight, cost-effective, and modular solution. The DREAM implant system is designed to weigh less than 10% (~2.1 g) of a mouse's typical body weight, ensuring animal welfare and minimal impact on behavior. Validation of the DREAM implant design shows minimal impact on behavioral key metrics such as locomotion - which can be significantly impacted in rodents when loads are placed on the cranium. This can benefit experimental paradigms that utilize freely moving as well as head-fixed animals by boosting animal well-being and allowing more ethologically sound experiments.
The system includes a microdrive for flexible adjustment of recording depth up to 7 mm and can be adapted to different types of probes and recording devices, providing researchers with a cost-effective and versatile tool for various experimental applications. The system is routinely combined with a metal microdrive21, which offers consistent probe recovery compared to other systems (expected average recovery rate: approx. three reliable reuses per probe) and drastically reduces the cost of individual experiments.
The design features a 3D-printed protective Faraday cage, allowing for cheap yet robust protection from electrophysiological noise, mechanical impacts, and infectious materials, enabling stable and noise-free recordings that suffer from minimal infection rates. This implantable cage consists of the so-called 'crown', designed for impact protection and to provide structure for the conductive metal mesh coating of the Faraday cage, and the crown ring, which serves as a mount for an implantable amplifier and/or probe connector (see Figure 1).
Finally, the headplates included in the modular implant system are designed to be compatible with a novel, efficient head-fixation system without adding extra bulk to the implant. In contrast to other existing systems, it does not require tightening small screws close to the implant, speeding up the fixation of mice in the experimental setup, and improving the experimenter-animal relationship, as well as behavioral adherence. At the same time, the headplate is used as a base on which to build the other modules of the DREAM chronic electrophysiology system.
Design files for the DREAM implant are published as open-source hardware at https://github.com/zero-noise-lab/dream-implant/. In the following sections, the design and fabrication of the DREAM implant system will be described, its successful implementation in a mouse model will be demonstrated, and its potential applications and advantages compared to existing systems will be discussed.
Protocol
All experimental procedures were conducted according to the institutional guidelines of the Max Planck Society and approved by the local government's ethical committee (Beratende Ethikkommission nach §15 Tierschutzgesetz, Regierungspräsidium Hessen, Project approval code: F149-2000).
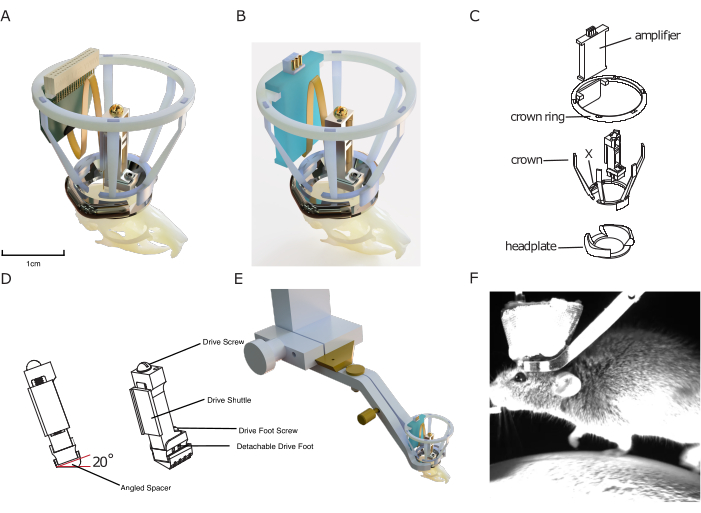
Figure 1: Implant design. (A) 3D rendering of the implant superimposed onto a mouse skull with a silicon probe connected to a probe connector. The central aperture of the headplate is approximately 10 mm for scale. The height of the drive is approximately 17 mm. The copper mesh that forms the outside of the Faraday crown, as well as ground/ref wires, is not shown. (B) Same as (A) with a connection to an amplifier board instead of a probe connector. (C) Exploded technical drawing of the implant, showing its components. (D) Rendering of an angled spacer that can be implanted underneath a microdrive, allowing to consistently implant the microdrive at a predefined angle (here: 20°). (E) Rendering of integrated head-fixation mechanism, showing implanted headplate with Faraday crown with the surrounding head-fixation clamp and the dove-tail connection to setup. (F) Image of mouse head-fixed on a treadmill using the implant's integrated head fixation mechanism. Please click here to view a larger version of this figure.
NOTE: Sections 1 and 2 discuss the pre-surgical preparations
1. Preparation of the silicon probe
- In case of probe reuse, clean the silicon probe according to the recommendations of the probe supplier (see point 10.12 for recommended procedure). Soak the probe in enzymatic cleaner (see Table of Materials) for 5-10 min, then rinse it in demineralized water. Do this as quickly as possible after explantation. A day before (re-)implantation, soak the probe in 70% ethanol for at least 30 min for disinfection.
- Measure channel impedances to make sure they are within specifications for the recorded signal. Follow the protocol for testing noise levels from the Neuropixels user manual22, measure impedance via the desired recording software (e.g., https://open-ephys.github.io/gui-docs/User-Manual/Plugins/Acquisition-Board.html#impedance-testing) and follow the target channel impedances from the silicon probe manufacturer or datasheet. If impedances are too high, consider recoating the electrode sites23.
- Solder a 0.05" solder tail socket (see Table of Materials) to the ground (GND) wire of the probe. Connect the socket to the GND pin (next step) during surgery.
NOTE: In this protocol, a separate reference (REF) pin is not used, as GND and REF are shorted on the headstage used. Therefore, only the GND pin will be mentioned in the remainder of the protocol. If a separate REF is used, repeat the following step for the REF pin. - To prepare the GND pin, repeatedly insert the pin side of a 0.05" solder tail socket (see Table of Materials) into the GND 0.05" solder tail socket until insertion is largely effortless. Using gold-plated pins can reduce the need for this smoothing step. This makes sure that the GND pin and socket can easily be connected during surgery without the need to apply excessive pressure, reducing the risk of injuries to the animal and probe damage.
- If an implantable pre-amplifier for the silicon probe is used, prepare them for chronic implantation following the supplier's procedures. Then attach the amplifier/connector to the ring of the Faraday cage by using silicone plaster to glue it to the area of the Faraday ring designed to hold the amplifier (see Figure 1).
NOTE: Preparing the implantable pre-amplifier for the silicon probe for chronic implantation following the supplier's procedures might include coating them in silicon or epoxy to avoid moisture damaging the electronics, as well as repeatedly mating the amplifier connector to reduce mating force when connecting the amplifier to the recording system during recordings. This is especially useful for Omnetics users.
2. Preparation of the microdrive and headgear
- Turn the screw on the microdrive body so that the microdrive shuttle is almost entirely retracted upwards.
- Optionally, attach an angled spacer (see Figure 1D) to the bottom of the microdrive with cyanoacrylate glue or dental cement, which can be used to allow for a specific degree of tilt to be used, for example, when recording through cortical layers in a region within the central sulcus, or within deep structures that may require a non-perpendicular approach (for angled spacer, see Table of Materials).
- Lay the microdrive horizontally onto the microdrive holder (Supplementary Figure 1).
- Place a small piece of adhesive putty (see Table of Materials) on the microdrive holder at a distance above the microdrive at which the head-stage connector will be placed. This distance depends on the length of the flex cable that connects the silicon probe to the headstage connector.
- Place a tiny drop of silicone plaster (see Table of Materials) onto the shuttle.
- Take the silicon probe out of its packaging with the help of a blunt, soft-tipped forceps. Make these by coating standard needle-nose forceps with 3 mm diameter heat-shrink tubing (see Table of Materials). Place the probe with the flex cable first onto the shuttle of the microdrive so that the bottom edge of the flex cable hangs slightly over the bottom edge of the microdrive shuttle.
- Gently pull the flex cable towards the top of the microdrive until the bottom edge of the flex cable meets the bottom edge of the microdrive shuttle. Make sure to push the flex cable against the left edge of the microdrive shuttle during this step so that it is placed exactly vertically on the microdrive at the end. At this point, ensure that the shanks of the silicone probe do not (or only minimally) protrude past the lower edge of the microdrive (depending on the exact length of the probe shanks and the depth of the targeted brain area).
- Place the head-stage connector of the probe onto the adhesive putty at the top of the holder to protect the probe from falling off.
- Use a 27 G syringe needle to apply a small drop of cyanoacrylate glue (see Table of Materials) between the flex cable and shuttle to secure the probe in place. Ensure the glue does not run onto the microdrive or along the flex cable beyond the shuttle (This is very important)
- Once the flex cable is glued in position, attach the amplifier to the crown ring (see Table of Materials) using silicone plaster. Then, attach the flex cable to the amplifier and cover the connection and cable in a thin layer of silicone plaster.
- After 5 min, when the plaster is set, store the microdrive and probe safely until further use.
- Cut pieces of copper mesh (see Table of Materials) into an open donut shape (see cutting pattern in Supplementary Figure 2) to cover the Faraday cage.
- Fasten the copper mesh cut-out onto the Faraday cage with small drops of epoxy resin (see Table of Materials). For this step, one can also replace epoxy with dental cement.
NOTE: The Faraday cage contains a space to house a probe connector or amplifier. This space is marked by an X in the design file, and it contains a supporting base for the amplifier/connector, as well as a larger distance between the two adjacent spokes of the cage. To create sufficient space around the amplifier/connector, fix a small amount of extra mesh between the two adjacent spokes, creating a protrusion. This ensures that the amplifier/connector can later be positioned in this 'pocket' without touching the Faraday cage. To ensure secure adhesion with minimal warping, use the crown ring placed directly on the crown to maintain shape and to support the thin spokes of the crown. Furthermore, use soldering helping hands to secure the crown and mesh during drying. If one struggles to maintain the shape of the crown when undergoing the procedure, attempt to epoxy only two of the crown arms at a time to prevent warping. - If separate grounding of the Faraday cage is desired, solder a small header pin onto a 30 mm grounding wire (see Table of Materials), then use conductive epoxy to adhere the wire to the copper mesh cut-out.
NOTE: This step is not adhered to in the lab. - At this point, store the prepared parts safely, and perform surgery at a later stage.
NOTE: Sections 3-6 discuss the implantation of the microdrive and headgear.
3. Surgery: Preparation of probe and workspace
- Sterilize and place surgical instruments in the surgical workspace following an approved procedure.
NOTE: This can include using a bead sterilizer, autoclaving instruments, or rinsing with 30% peroxide or 90% ethanol, depending on the approved experimental protocol. - Place the ceramic dish used to prepare the dental cement in an ice box, fridge, or freezer, following the instructions in the dental cement kit (see Table of Materials). Use the cooled ceramic dish during cement mixing to increase the time the cement is malleable. Use a cooled dish whenever longer cementing steps are required.
- If histological verification of probe placement at the end of the experiment is desired, extend the silicon probe right before the surgery by turning the screw on the microdrive counterclockwise and apply a lipophilic dye (see Table of Materials) to the probe by dipping it in a small drop of the dye. Prepare the lipophilic dye from a commercially bought dimethyl sulfoxide (DMSO) or ethanol (EtOH) diluted stock solution (see Table of Materials) by diluting it in a suitable buffer such as PBS at a 1-5 µM concentration.
4. Surgery: Preparation of the animal
- Follow an approved anesthesia protocol for a 2-4 h rodent surgery under aseptic conditions. This can include general and local anesthesia, analgesia, application of eye ointments, and injections of saline. Here, use injectable anesthesia (ketamine 100 [mg/kg]/medetomidine 0.5 [mg/kg]) together with local analgesia cream and eye ointment (see Table of Materials), and place the animal on a heating pad to regulate body temperature. Follow an approved monitoring procedure, e.g., toe pinch, to monitor the depth of anesthesia at regular intervals (every 20 min) throughout. Monitor breathing for regularity and depth to ensure the correct plane of anesthesia is not exceeded.
- When the animal is fully anesthetized, move it to a separate non-sterile shaving area.
- Ensure that the animal is warmed sufficiently; for example, place it on a heating pad. Remove hair on the top of the skull. Do this with an electric shaver or depilation cream (see Table of Materials) or by repeatedly shaving the top of the head with a scalpel covered in 70% ethanol.
- Carefully remove loose hairs to make sure they do not get in contact with exposed tissue later. To remove hairs, use, e.g., tissues wetted with 70% ethanol and/or a squeeze ball pump. If using depilation cream, ensure that this is removed using cotton swabs and saline thoroughly.
- Disinfect the shaved area multiple times with an iodine-based disinfectant (see Table of Materials) and alcohol using cotton swabs, moving from the center of the head to the sides to brush any remaining loose hairs away from the incision site.
- Apply betadine to the shaved area around the incision and place a drape over the body and unshaved area of the animal. Optionally, apply a drape directly to the incision area. This ensures a sterile working area and protects surgical instruments and materials from coming into contact with unsterile fur.
- Place the animal in a stereotactic frame using ear bars and nose holder (see Table of Materials). Ensure the animal is in an adequate plane of anesthesia before starting the incision by using methods such as the toe pinch test and monitoring respiratory patterns.
- Using small surgical scissors (see Table of Materials), cut an almond-shaped opening in the skin on top of the skull, reaching from just the posterior of the lambda suture to between the eyes.
- Remove the subcutaneous membrane and periosteum by cutting away while still wet, then scratch the skull with a scalpel blade to remove soft membrane tissue on the skull's surface that may impede dental cement's adhesion.
- Optional: Once the skull has been cleared of membrane tissue, briefly apply a thin layer of 0.5% peroxide and wash it off with water-based iodine disinfectant (e.g., Betadine) before roughening the surface of the skull to improve adhesion of the primer to the skull.
- Carefully roughen the surface of the skull by scratching a crisscross pattern with the tip of the scalpel turned upside down. This helps dental cement to adhere to the skull later.
NOTE: Do not scratch too vigorously on top of sutures since this can cause the sutures to rupture and leak intracranial fluid, which impairs adhesion of the dental cement. - Alternate between scalpel blade and sterile cotton buds to gently scratch/push away neck muscles attached to the sides of the lambda suture until the muscles have been pushed back to the 'edge' of the skull on top of the cerebellum. This helps to minimize muscle noise in neuronal recordings.
- Fill a 1 mL syringe with a 27 G needle (see Table of Materials) with small amounts of surgical cyanoacrylate glue (see Table of Materials). Then, glue the skin to the skull edges using the syringe to smear tiny drops of superglue across it. Glue tissue as flat as possible to the skull to leave space for implants. This procedure ensures that skin and muscles do not come in direct contact with parts of the implant, which avoids muscle noise in recordings and improves the adhesion of the dental cement.
- Apply dental cement primer across the skull for extra adhesion and harden with UV light (see Table of Materials). This improves dental cement adhesion and prevents cranial sutures from leaking and weakening the cranial-cement bond over time.
- Find the target location for the probe implantation relative to bregma or lambda and outline the craniotomy around it with a surgical marker. Place the headplate on the skull so that the craniotomy lies within it, with space for the microdrive at one side of the craniotomy, as well as for 1-2 grounding pins.
- Implant the headplate using dental cement. Mix dental cement in the designated cooled ceramic dish (see step 3.2). Ensure the headplate adheres to the skull on all sides, forming a watertight 'well'.
- With a dental drill (size US ½ HP), drill a small burr hole the width of the header pins prepared in step 1.4 over the brain area(s) to be used as GND/REF. If grounding the Faraday cage is desired, drill another small burr hole close to the edge of the Faraday cage for the Faraday-GND header pin.
NOTE: For the GND/REF header pin(s), place the craniotomy at a sufficient distance from the edge of the cage so that the header pin itself can be placed within it later without touching the Faraday cage. - Clean the craniotomy by gently dripping sterile saline onto it with a syringe and removing it with non-shedding wipes (see Table of Materials). Repeat until all blood and loose tissue is removed.
- Prepare a 0.7% agar (see Table of Materials) solution in saline, cool it slightly, and introduce it into the craniotomy using a 27 G needle on a 1 mL syringe.
- Gently insert a GND pin (see step 1.3) into each craniotomy drilled in the previous step. The pin(s) will be surrounded by agar on all sides (see step 4.17). Apply cement around the header pins to secure them and provide electrical isolation.
- Clean the ceramic dish and place it back in the fridge/freezer.
- With a dental drill, drill the outline of a larger craniotomy (circular or square) by moving around the edge in steady movements. Ensure that the craniotomy is 1 mm x 1 mm to 2 mm x 2 mm to allow for small adjustments to the placement of the probe to avoid blood vessels without exposing too much of the cortex. If possible, avoid placing craniotomies over sutures. Drill in rounds of 20-30 s, and cool down the skull with saline between drilling rounds.
NOTE: When beginning drilling, it is useful to mark out the leading edge of the microdrive with a marker, hence ensuring that when drilling, a straight edge can be formed in parallel to the microdrive leading edge. This improves the chances of avoiding cement in the craniotomy when fixing the microdrive in place, as well as improving adhesion, preventing microdrive overhang over the craniotomy and allowing for greater lateral maneuverability when placing the microdrive in relation to the final recording site position. - After a few initial rounds of drilling, test the resistance of the drilled-out portion of the bone by gently pushing on it with fine forceps (size 5 or finer; see Table of Materials).
- Keep testing between drilling rounds until the bone begins to 'bounce' underneath the forceps when pushed. When this is the case, add a drop of saline on top of the craniotomy to soften up the bone, then use the forceps to gently remove the drilled-out piece of bone.
- If the bone cannot be removed gently, do another round of drilling, focusing on the points where the bone is still attached more strongly. In general, aim to remove the skull with gentle pressure from the forceps before it has been entirely drilled through since this typically minimizes tissue damage.
NOTE: Ensure the surface of the dura is moistened regularly, both during drilling to reduce temperatures and following bone flap removal. This improves the chances of easy probe insertion by preventing the dura from drying out and becoming more challenging to penetrate. If the dura proves to be too tough to penetrate, or blunt or multi-shank probes are being used, a durotomy is performed by lifting the dura with a 27 G needle and performing a small incision under saline immersion to prevent the dura from sticking to the brain surface.
- Cover the craniotomy with a hemostatic sponge (see Table of Materials) soaked in cool, sterile saline to protect the dura and brain.
5. Surgery: Probe implantation
- Attach the custom microdrive holder (see Table of Materials) to the arm of the stereotactic apparatus. If the microdrive was removed from the microdrive holder after probe preparation, place the microdrive with the attached silicon probe into the microdrive holder.Angle the stereotax arm as required to reach the desired target brain area. Place the crown ring with the attached amplifier onto the three vertical pins at the rear of the microdrive holder (see Supplementary Figure 1).
- Lower the microdrive to within ~0.5 mm of the craniotomy, then use forceps to connect the GND/REF header pin(s) attached to the probe to the corresponding GND/REF pin(s) implanted on the skull (see steps 4.14-4.15). See Supplementary Figure 3 and Supplementary Figure 4 for examples of drive, craniotomy, and GND/REF pin placement.
- Once in place, optionally secure the pin(s) with a drop of conductive silver epoxy (see Table of Materials) for a more robust connection. Once silver epoxy is cured, cover the connected pins in a small amount of dental cement (see Table of Materials) to ensure the connection stays stable over long periods and that there is no electrical connection with the surrounding tissues and/or implant elements.
- Remove the hemostatic sponge from the craniotomy (see step 4.22).
- Position the stereotactic arm with the microdrive over the craniotomy.
NOTE: If the probe is retracted, make sure that the microdrive is placed in a way that the probe would touch down on a part of the craniotomy that does not contain large blood vessels. - Lower the microdrive, if necessary, by adjusting the location and angle until the probe shank touches the dura or brain surface (see step 4.21) in the target area.
- Mix dental cement in the designated ceramic dish (see step 3.2), and cement the base of the microdrive in place, focusing on the three sides of the microdrive base that are not facing the electrode. Ensure the cement does not touch the microdrive above the removable 'base' (see Figure 1D).
- Make sure that any space between the base and skull is covered fully with dental cement. Clean the ceramic dish and put it back in the fridge/freezer. Wait for the cement to cure, approximately 10-15 min.
NOTE: A small gap is left between the microdrive base and skull, and the cement is used in its most fluid form to fill it. Once the cement has thickened slightly, the cement between the walls of the microdrive base and the skull is built up. Very small amounts of cement are always used, as the flow of the substance can be unpredictable, and larger volumes may flow into undesired regions. Small amounts of hemostatic sponge dipped in saline can be used to cover portions of the craniotomy. If cement should accidentally flow onto the craniotomy, remove the cement with forceps once it enters a film-like consistency.
- Make sure that any space between the base and skull is covered fully with dental cement. Clean the ceramic dish and put it back in the fridge/freezer. Wait for the cement to cure, approximately 10-15 min.
- Lower the silicon probe onto the brain, carefully monitoring the probe position through a microscope. When the probe shanks touch the brain, lower the probe quickly by ~250 µm (one full turn of the screw is 282 µm) to ensure that the probe breaks through the resistance of the dura/cortical surface.
- Verify this visually. If the probe has not broken into the cortex, wait for 5 min, then attempt to etch through the dura with the shank tip by repeatedly raising and lowering the probe by a few tens of micrometers while the dura/cortex is under tension from the probe tip.
- Once the probe has broken through the surface of the cortex, gradually lower it at a slower pace (100-200 µm /min) until either the target coordinates are reached or the probe has moved by more than 1000 µm. If the target requires the probe to move by more than 1000 µm, advance the probe in steps of maximally 1000 µm/session over the following recording sessions until the target coordinates are reached.
NOTE: Skip this step if monitoring neuronal signals while lowering the silicon probe is preferred. Steps for this are described in section 7. - Prepare silicone elastomer according to instructions (see Table of Materials) and dispense a small drop into the craniotomy using a 1 mL syringe (see Table of Materials).
- Once dry, cover the silicone elastomer with a 50/50 mix of bone wax and mineral oil. This step further protects the probe and prevents the accumulation of debris and dry plasma over the craniotomy, making extraction simpler and safer. Exercise caution, as working around the probe while it is lowered can lead to breakage.
6. Surgery: Implantation of Faraday cage
- When the dental cement has fully solidified, loosen the microdrive holder by loosening the lateral screw fixating the drive with an Allen key (see Supplementary Figure 1). Gently retract the holder by ~1 cm so that the microdrive is free-standing, but the probe amplifier/connector remains fixed to the implant holder without stretching the flex cable.
- Place the pre-made crown and Faraday mesh around the headplate by stretching the cage at the opening and slotting it over the microdrive and Flex-cable horizontally, then fix it onto the headplate with dental cement.
NOTE: Make sure to close all spaces between the Faraday cage and skull with dental cement to protect the implant from contamination. - Put the Faraday crown ring (see Table of Materials) with probe connector/headstage over the crown, aligning the integrated holder for the probe amplifier/connector with the area marked by an indented 'X' on the Faraday crown (see step 2.13).
- Secure the ring to the Faraday cage with a small drop of cyanoacrylate glue or dental cement at each spoke-ring junction.
- Once the Faraday ring with integrated probe amplifier/connector is secured in place, fully retract the stereotactic arm with the microdrive holder. See Supplementary Figure 3 for a step-by-step guide on the assembly of these components.
7. Post-surgery test recording
- Connect the probe amplifier/connector to the recording hardware and start a recording.
- If the probe has not yet reached its target location during the initial insertion (see step 5.9), slowly turn the microdrive screw counterclockwise to lower the probe while monitoring neuronal signals.
NOTE: Signals should change a) when electrodes touch the layer of silicone elastomer above the craniotomy, and b) when the electrodes begin to move into the brain (see step 7.2). High-frequency neuronal activity will be registered by electrodes that are fully inserted in the brain, while electrodes that are in contact with the CSF on the brain surface will typically show a low-pass-filtered neuronal population signal without spiking activity (akin to an EEG trace), and recording sites in air will register increased electrical noise. It is possible to additionally verify the probe insertion depth by measuring the impedance of individual channels after the test recording. Channels in contact with air should show high impedance (indicating an open circuit) and impedances like the ones measured before surgery for the channels touching CSF or already in the brain. Advance the silicon probe by a maximum total distance of approximately 1000 µm per session, with a maximum speed of approximately 75 µm/min (see step 5.5). - When neural local field potentials are visible across the probe and/or the probe is advanced by a maximum of 1000 µm, end the test recording and disconnect the head-stage connector.
8. Recovery
- Cover the Faraday cage with self-adherent veterinary wrap (see Table of Materials).
- End anesthesia and let the animal recover whilst administering post-operative care for several days following approved experimental guidelines. This may include administering analgesia and prophylactic antibiotics.
NOTE: This study used injectable buprenorphine (0.1 mg/kg), injectable enrofloxacin (20 mg/kg), as well as enrofloxacin (0.17 mg/mL), and metamizole (1.25 mg/mL) in the drinking water for 3 and 5 days post-surgery, respectively. Depending on water intake, mice will receive ~1.02 mg/day of enrofloxacin and ~7.5 mg/day of metamizole. - If the electrodes on the silicon probe are not yet at the desired target location, turn the screw of the microdrive in small steps with a maximum of four full turns (or ~1000 µm) per session. If necessary, repeat this procedure over several days until the target is reached. Combining the probe movement with simultaneous recordings to evaluate electrophysiological activity in areas transversed is recommended.
NOTE: The implant was designed to require very little aftercare and maintenance post-implantation. However, due to the positioning of the head plate close to the neck, skin movement may cause irritation. Monitor this area and apply antibiotic cream if irritation occurs, following approved experimental guidelines.
9. Behavioral experiments and chronic recordings
- For chronic head-fixed recordings during task performance, attach the headplate at the base of the Faraday cage to the head-fixation clamp by manually opening the clamp and clamping the implanted headplate (see Figure 1C, E, F).
NOTE: If head fixation is not needed, this implant system can also be used for freely moving recordings. For freely moving recordings, skip steps 9.1 and 9.7. - Remove the self-adherent veterinary wrap from the implant.
NOTE: To minimize discomfort for the animal, it is suggested that a simple, rewarding behavioral task be started before this step as a distraction while the experimenter works with the implant. - Attach amplifier/connector to recording equipment.
- Conduct neuronal recordings as the animal performs the task.
NOTE: If the goal is to maximize the number of extracellular units recorded, move the shuttle by a few tens of micrometers whenever the neural yield in a location decreases. Note that after moving the probe, the signal can take minutes to hours to stabilize. Therefore, it might be beneficial to move the probe at the end of a session so that the signal can recover until the start of the next session. - Disconnect the recording equipment and cover the implant in a new veterinary wrap at the end of the behavioral recording.
- Open the head-fixation clamp to detach the animal from the head fixation.
10. Probe recovery
- At the end of the final recording, retract the silicon probe as far as possible onto the microdrive by turning the screw clockwise. Do this while the animal is head-fixed and behaving or with the animal anesthetized in the surgical setup. Chart the exit of the probe from the brain by monitoring neuronal signals simultaneously and checking for the signature of electrodes being immersed in the brain, touching the brain surface, or in contact with air (see step 7.3).
NOTE: Depending on the histology protocol and probe, electrolytic lesions are performed before retracting the probe to determine the exact location of some electrodes on the probe. If monitoring the probe exit via neuronal recording is not necessary, retracting the probe once the animal has been terminated is also possible. - Terminate the animal following approved guidelines (this includes perfusing the animal if fixating the brain for subsequent histology is planned).
- Wait for ~ 10 min after the animal has died. Then, head-fix the animal in the stereotax, making sure that the animal's head cannot move during explant to prevent probe breakage.
- Apply a drop of saline on top of the craniotomy and let it soak for a few minutes to soften dried biological tissue on the probe shank and lower the chance of shank breakage.
- Place the stereotactic holder approximately 0.5 cm above the microdrive. Then cut the upper end of the spokes of the Faraday cage with small surgical scissors (see Table of Materials) to free up the Faraday ring holding the amplifier/connector and transfer the ring back onto the vertical pins at the top of the stereotactic holder (see step 5.1 and Supplementary Figure 1).
- Carefully cut away the copper mesh with the same surgical scissors by cutting out u-shaped areas of mesh between the spokes of the Faraday crown. Then, cut off the crown's plastic spokes at the base.
NOTE: Avoid bending the printed plastic spokes as they are being cut, as they may snap and send plastic debris flying toward the probe. - Lower the stereotactic holder until the microdrive can be fixated in the holder using the holder's lateral screw, fixate the microdrive, then loosen the T1 screw that connects the microdrive body to the microdrive base.
- Slowly retract the stereotactic arm with the implant holder to lift the microdrive off its base. Ensure that the microdrive separates from the base at a perpendicular angle (i.e., 'vertically' from the base).
NOTE: If the microdrive body and base do not separate easily, verify that the movement of the stereotactic arm is not at an angle compared to the microdrive orientation. If necessary, the holder and microdrive are re-aligned to each other by slightly loosening the fixation of the animal's head and repositioning it accordingly. Correct alignment is one of the crucial aspects for easy recovery of the microdrive. Also, check whether there is any residual dental cement connecting the microdrive and microdrive base (see step 5.5). If so, the cement is carefully scraped off with a scalpel and/or dental drill depending on the amount of cement used. - Raise the stereotactic arm with the attached probe to create sufficient space below it.
- Remove the animal from the stereotax, and prepare the brain by following an approved histology protocol if desired. Recover the implanted microdrive base and clean it by soaking it in acetone for several hours for later reuse.
- Place a clean microdrive base on adhesive putty (see Table of Materials), then lower the microdrive onto the base and tighten the screw. To prevent breakage, monitor the probe position under a microscope throughout the process. This step can be completed at a later time if the implanted microdrive base needs to be cleaned for reuse first.
NOTE: This protocol calls for the use of adhesive putty as a platform for the base, which is vital as it both secures the base whilst also having a degree of give, ensuring the base does not slip and collide with the probe. The putty should be shaped into a vertical' cliff face' on the side of the microdrive base where the probe will be lowered. This ensures that if the probe is lowered past the base, it does not make contact with the putty underneath. The putty 'tower' should also be tall enough that if it is lowered past the microdrive base, the probe does not make contact with the table surface on which the putty is placed. Finally, secure the putty well to the surface to prevent it from slipping or falling. When lowering the microdrive onto the microdrive base held by the putty, ensure a side profile view from the microscope to monitor the progress so that as the probe is lowered, it does not collide with either the base or the putty. - Clean and sterilize the probe following the manufacturer's instructions. For the most commonly available probes, soak them in an enzymatic cleaner (see Table of Materials) for 12 h, then rinse in demineralized water and sanitize in alcohol. Do this by lowering the probe into a large beaker containing the enzymatic cleaner while still attached to the microdrive holder on the stereotactic arm.
NOTE: If desired, measure the impedances of the electrodes on the probe after cleaning to monitor the potential degradation of individual electrodes. - Store the microdrive with the cleaned probe safely until the next experiment.
Representative Results
This protocol presents a chronic implantation system that enables researchers to implement lightweight, cost-effective and safe chronic electrophysiology recordings in behaving mice (Figure 1). The main factors that determine successful application of this approach include: complete cement coverage of the skull, a minimally invasive and properly protected craniotomy, secure attachment of the microdrive and wiring to the skull and complete continuity of protective Faraday material. When these points are accounted for, high-quality recordings can be reached consistently. Here representative results pertaining to the following main aspects of surgery success are shown:
1) Is the implant interfering with animal behavior or well-being?
2) Is signal quality high, and can signals be maintained over prolonged periods of time?
3) Can recordings be combined easily with task performance?
To assess the impact of the implant on animal behaviour, we analysed tracked locomotion patterns in five implanted animals. Figure 2A shows an example of an animal freely moving inside of a play cage for 10 min before and 1 week after implant. One can see that movement patterns are unchanged. This observation is confirmed by Figure 2B, C showing the distributions of movement speeds and head directions across animals. Both running speed and head directions were largely unchanged before and after implantation, and if anything, running speeds seemed to be slightly elevated after surgery. Supplementary Video 1 shows a short video recording of an animal 6 days after implantation surgery. Typical home cage behaviors like locomotion, grooming, rearing and foraging in the home environment are all visible and indicate successful recovery from surgery, as well as general health. The low behavioral impact of the implant is most likely due to its low weight and manageable height.
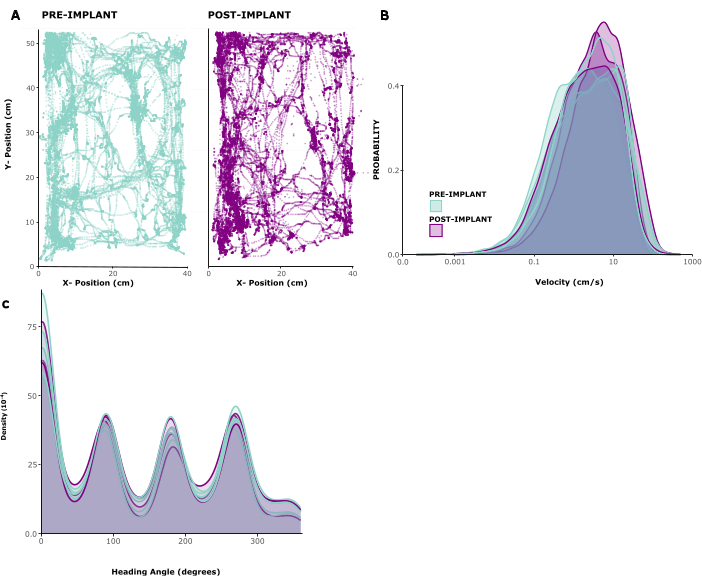
Figure 2: Locomotion before and after surgery. (A) Example locomotion of an animal before (left panel) and after (right panel) implantation. x/y coordinates are in centimeters, points show position of the animal at each timepoint over a period of 10 min. (B) Distribution of movement speeds in cm/s for 5 sessions before and 3 sessions after implantation in 5 animals. (C) Kernel density for probability of movement in different directions, for the same sessions analyzed in(B). Please click here to view a larger version of this figure.
Next, the signal quality in Local Field Potential (LFP) and spiking activity across recording sites is assessed. Here, we show representative data from cortical recordings in the primary visual cortex (V1). For validation, putative single-unit activity was extracted from broadband neuronal signals recorded in V1 of an awake mouse using Kilosort 3 (see Figure 3). Figure 3A shows the location of extracted single units on the probe shank, Figure 3B shows the corresponding spike waveforms, and Figure 3C shows the spiking responses of the same neurons to a current source density (CSD) protocol. In this paradigm, widefield flashes were presented with a duration of 300 ms at a frequency of 1 Hz (i.e., 300 ms on, 700 ms off) over 200 trials. Finally, Figure 3D shows the same units' responses to a visual receptive field mapping protocol, consisting of 2000 frames of randomly selected black and white squares on a grey background, and each presented for 16.6 ms. Squares covered 12 degrees of visual angle each and were selected from a field of 15 x 5 possible locations so that the mapping paradigm covered a visual space of -90 to +90 degrees azimuth and -30 to +40 degrees elevation in total. Firing rate responses to each stimulus frame were extracted by analyzing the maximum firing rate across a 16.6 ms window, subject to a delay of between 40-140 ms, identified as optimal per channel based on the maximum activity in each window. This type of recording can be used to guide adjustment of the insertion depth of each electrode and to assess signal quality after the implant surgery.
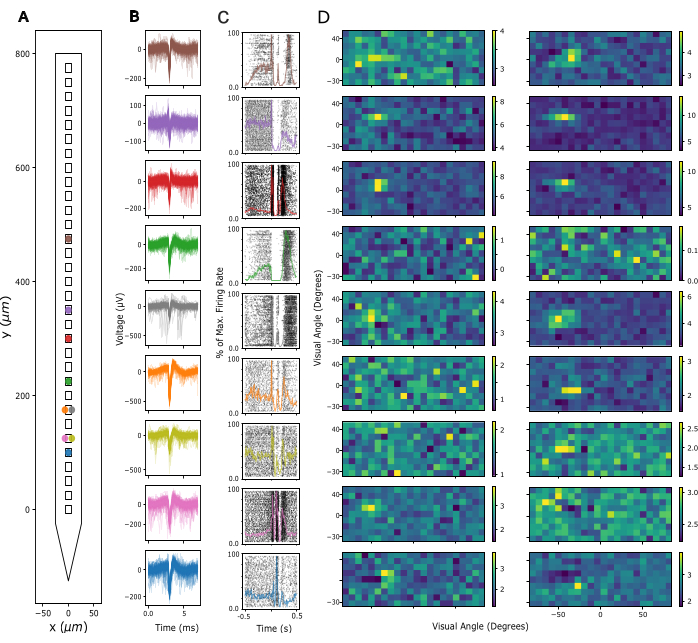
Figure 3: Recorded neuronal signals. (A) Inferred location of single units sorted by Kilosort 3 spike sorting package along the probe's electrode contacts. (B) Spike waveforms for the same units shown in A across 5 ms of time. Thin lines: Individual spike waveforms. Thick lines: Average spike waveform. (C) Raster plot of spikes in response to a current source density (CSD) paradigm presenting 300 ms widefield flashes followed by a 700 ms black screen. Responses are shown for the same units as in A and B. Superimposed colored lines represent peri-stimulus time histograms (PSTHs) of the same responses. Firing rates for the PSTHs were calculated in 10 ms bins and then normalized by the maximum firing rate across the entire PSTH. Time 0 is centered around the widefield flash stimulus. (D) Estimated receptive fields of the same units as in A-C, measured by a Sparse Noise Receptive Field Mapping paradigm. Each plot shows average firing rate activity over a 16.6ms analysis window in response to the onset (left panel) or offset (right panel) of white and black square stimuli. Stimuli were presented for the duration of 16.6 ms, located randomly across a 5 x 15 square grid spanning 180 degrees of visual angle horizontally and 70 degrees of visual angle vertically. Firing rate activity was z-scored across the entire receptive field grid (see color bar). Please click here to view a larger version of this figure.
Recording quality remained high across repeated recordings for weeks to months. Figure 4A shows longitudinal LFP recordings from one animal over 15 weeks. LFPs were recorded in response to the CSD paradigm described above (see Figure 3A-C). Figure 4A shows averaged LFP responses 500 ms following flash onset. In this example, we used a linear probe with 32 channels, with an interelectrode distance of 25 µm. Note that on day 18, the probe depth was adjusted, shifting the probe downwards by 600 µm. Both before and after this adjustment, LFP signals remained stable across recording days.
Consistent with this, spike waveforms of putative single units were discernible over many recordings. Figure 4B shows representative example spike waveforms from three recording sessions across a month of recordings, demonstrating that single unit activity can be identified successfully over time. Figure 4C shows the overall number of putative single units extracted from chronic recordings in six animals, spanning a window of up to 100 days. Single units were defined according to the default criteria of kilosort 3.0 (see Supplementary Table 1). As one can see, the number of clearly defined single units typically amounted to ~40 in the first-week post-implantation, and then dropped off gradually, moving towards an apparently stable asymptote of ~20 units. Given that these recordings were conducted using linear 32-channel probes, this equates to an expected yield of about 1.25 single units per electrode directly after implantation, declining to approx. 0.65 single units per electrode in long-term recordings. Repeated connection to the implant's amplifier/connector over sessions did not appear to impact either recording quality or implant stability since the Faraday crown that holds the amplifier/connector can withstand repeated forces of over 10 Newton, an order of magnitude larger than even the maximal mating forces required by standard connectors (see Supplementary Video 2).
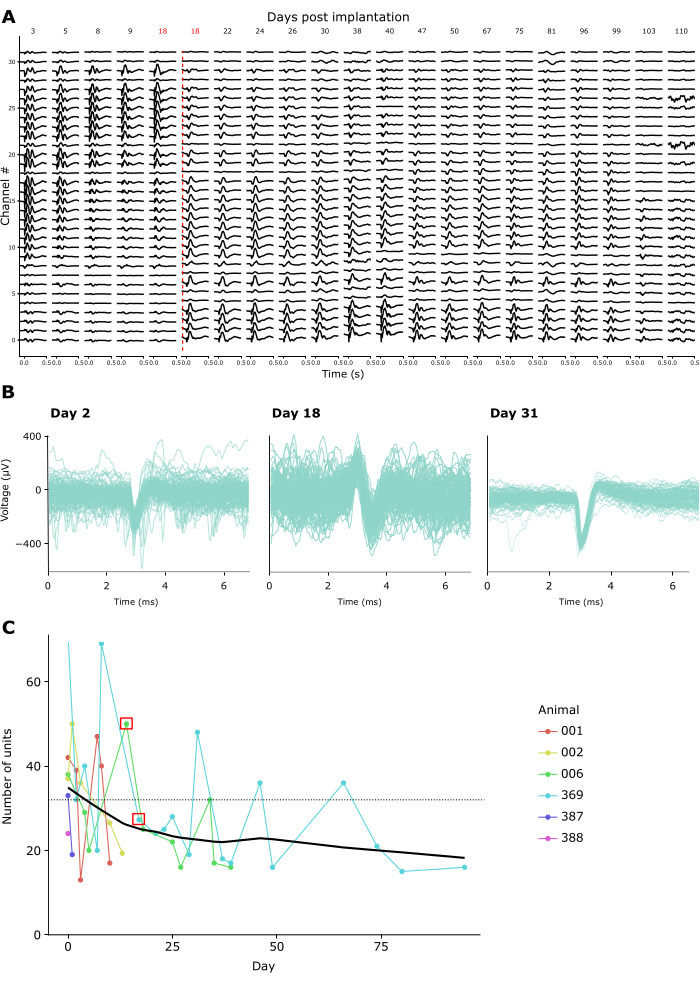
Figure 4: Stability of neuronal recordings over time. (A) Average LFP activity in response to a widefield flash CSD stimulus, shown across all 32 channels of a chronically implanted probe from 3-110 days post-implant. The red vertical line denotes the probe being lowered to a new location due to channels 0-8 recording from outside the brain by Day 18 post-surgery. (B) Spike waveforms of three example units from the same chronic implant recorded repeatedly across four weeks. Thin lines: Individual spike waveforms. Thick superimposed line: Average spike waveform. (C) The number of putative single units detected by Kilosort 3 across recording days for 6 animals (see inset legend). The red square denotes the days when the probe was moved. The dotted line denotes the number of electrodes per implant used in these recordings (32). Please click here to view a larger version of this figure.
Finally, by providing a modular system including a microdrive as well as a wearable Faraday cage and a headplate that doubles as an implant base and a device for head-fixation, this protocol enables the integration of chronic electrophysiology with head-fixed behavior. Here, example data from mice traversing a virtual environment on a spherical treadmill are shown. Figure 5A shows running-related spiking activity of 20 units in an example trial. Figure 5B shows the diverse but robust relationships between running speed and spiking activity of individual spike-sorted units, as well as a population average for the same effect in Figure 5C, confirming the well-established effect of locomotor activity on neuronal activity in rodent V124.
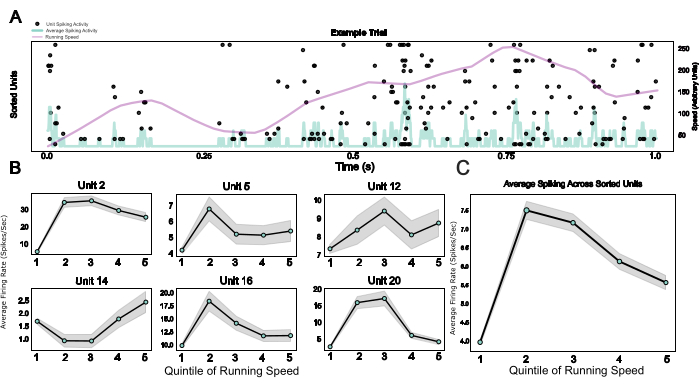
Figure 5: Neuronal responses during head-fixed behavior. (A) Raster plot of single unit responses across an example trial, with running speed (purple line) and average firing rates across all single units (light blue line) superimposed. (B) Single unit activity during different running speed categories, shown for six example units. (C) Average spiking activity across all single units in one example session, plotted across the five quinitiles of the running speed distribution. Running speeds in this session ranged from 0 to 0.88 meters/second. Please click here to view a larger version of this figure.
Supplementary Table 1: Table showing default parameters used by Kilosort 3 when identifying single units in the recordings shown in Figure 3, Figure 4, and Figure 5. Please click here to download this File.
Supplementary Video 1: Video showing animal locomotor activity post implant. Video taken after 5 day recovery phase is complete, showing normal locomotor behavior, as well as adaption to the size and weight of the implant. The animal can be seen normally exploring a play cage containing environmental enrichment. Please click here to download this File.
Supplementary Video 2: Video showing force being applied onto the assembled Faraday crown. The forces withstood by the Faraday crown are approximately one order of magnitude larger than the connection force needed for standard connectors such as 4-pin polarized nano connectors. Please click here to download this File.
Supplementary Figure 1: Figure showing images of the drive holder. Printable design files can be found in the corresponding Github repository (https://github.com/zero-noise-lab/dream-implant/). Please click here to download this File.
Supplementary Figure 2: Template for copper mesh. Print the template with the original scaling and use the stencil for cutting out the copper mesh (step 2.12). Use the scale bar for verifying and, if necessary, adjusting the scaling of the print. Please click here to download this File.
Supplementary Figure 3: Photo series showing the assembly steps of the implant during surgery. Two microdrives, as well as two amplifiers, are installed in this case. Please click here to download this File.
Supplementary Figure 4: Drawing of mouse skull featuring example placement of drives, craniotomies (in green), and GND/REF pin (in red). Pin location is suggested due to placement in the cerebellum, which is unlikely to interfere with cortical recordings. Please click here to download this File.
Discussion
This manuscript presents a protocol for the fast, safe, and standardized implantation of probes, which also allows probe recovery and reuse at the end of the experiment. The approach makes use of a modular system of implant components, specifically a microdrive, which is compatible with all common silicon probes and recording systems, a headplate that can be used for head-fixed behavioral experiments, and a wearable Faraday cage to protect the implant. This constellation allows users to flexibly adapt their implant to different experimental paradigms, such as head-fixed versus freely moving behavior or implant miniaturization (without Faraday cage) versus increased long-term signal robustness (with Faraday cage) - without having to sacrifice the standardization of the implant in the process.
This approach makes chronic electrophysiological recordings more standardized (through prefabricated elements that do not require assembly by hand), less costly (through probe recovery), less time-consuming (by simplifying surgery steps), and more easily compatible with animal welfare and behavior (through decreased implant size and stress-free head fixation). As such, this protocol aims to make electrophysiological implants in behaving rodents attainable for a broader range of researchers beyond the pioneering labs at the cutting edge of the field.
To achieve this aim, the protocol presented here minimizes the trade-off between several often equally crucial aspects of microdrive implants, namely flexibility, modularity, ease of implantation, stability, overall cost, compatibility with behavior, and probe reusability. Currently, available approaches often excel at some of these aspects but at a steep cost to other features. For instance, for use cases that demand absolute implant stability over long time periods, the best implant approach may be to directly cement the probe onto the skull25. However, this also prevents probe reuse, as well as repositioning of recording sites in case of bad recording quality, and it is incompatible with standardized implant placement. Similarly, while the AMIE drive provides a lightweight, low-cost solution for recoverable implantation of probes, it is limited to single probes and restricted in the placement of the target coordinates17. At the opposite end of the spectrum, some commercially available nano-drives (see Table 116,17,21,26,27,28,29,30) are extremely small, can be placed freely on the skull, and maximize the number of probes that can be implanted in a single animal16. However, they are expensive compared to other solutions, require experimenters to be highly skilled for successful implant surgeries, and prohibit probe reuse. The microdrive developed by Vöröslakos et al.21, a lightweight version of which is also part of this protocol, sacrifices small implant size for better ease-of-use, lower price, and probe reusability
Table 1: Comparison of popular strategies for chronic probe implants in rodents. Availability: whether the microdrive is open source (for researchers to build themselves), commercially available, or both. Modularity: Integrated systems consist of one or few components that are in a fixed relation to each other, while modular systems allow free placement of the probe /microdrive relative to the protection (head gear/Faraday cage) after production of the implant (e.g., at time of surgery). Modularity was determined from published information or implantation protocols of the listed implants. Headfix: Yes: The implant has mechanisms for head-fixation integrated into its design, X: The implant leaves the space to add an extra headplate for fixation without big issues, No: The design of the implant likely creates space issues or requires substantial design modifications for use with head fixation. Probe placement: Restricted: Probe location is limited at the implant design stage. Flexible: Probe location can be adjusted even during surgery. Number of probes: the number of probes that could be implanted. Note that implanting >2 probes on a mouse does pose a significant challenge independent of the chosen implant system. Probe reusability: yes, if the probes can, in theory, be reused. Weight/size: weight and bulkiness of the implant. Please click here to download this Table.
To create a system that reconciles these different requirements more seamlessly, the DREAM implant was designed on the basis of the Vöröslakos implant21, but with several fundamental modifications. First, to reduce overall implant weight, the microdrive used here is produced in machined aluminum rather than 3D-printed stainless steel, and the Faraday crown is miniaturized, achieving an overall weight reduction of 1.2-1.4 g depending on the choice of headplate material (see Table 2). Second, the headplate surrounding the microdrive was designed to allow for an integrated head fixation mechanism that enables fast and stress-free head fixation while doubling as a base for the Faraday cage, giving access to most potential target areas for neuronal recordings and adding only minimal weight to the implant. The flat shape of the fixation mechanism and lack of protrusions also ensure minimal impairment of animals' visual field or locomotion (see Figure 2A-C), a clear improvement over previous systems31,32. The Faraday crown and ring that are fixed onto the headplate were also substantially altered compared to previous designs. They now do not require any ad-hoc adaptation (e.g., in terms of connector placement) or soldering throughout the surgery, removing potential causes of implant damage and unpredictable variance in implant quality. Instead, the DREAM implant provides multiple standardized crown ring variations that allow placing each connector at one of four pre-defined positions, minimizing variability and effort during surgery. Finally, by optimizing the implant system for probe recovery, the DREAM implant allows experimenters to drastically cut the cost as well as preparation time per implant since the microdrive and probe can typically be recovered, cleaned, and reused together.
For a more exhaustive overview of the trade-offs posed by different implant systems, see Table 1. While the approach presented here does generally not provide maximal performance compared to all other strategies, e.g., in terms of size, stability, or cost, it operates in the upper range across all these parameters, making it more easily applicable to a wide range of experiments.
Three aspects of the protocol are particularly crucial to adapt to each specific use case: The constellation of ground and reference, the technique for cementing the microdrive, and implant validation via neuronal recording. First, when implanting the ground and reference pins, the goal was to identify the sweet spot between mechanical/electrical stability and invasiveness. While, e.g., floating silver wires embedded in agar are less invasive than bone screws33, they are likely more prone to becoming dislodged over time. The use of pins, coupled with agar, ensures a stable electrical connection whilst also having the advantage of being easier to control during insertion, avoiding tissue trauma. Ground pins cemented to the skull are unlikely to become dislodged, and in the event of the wire becoming separated from the pin, reattachment is usually simple due to the larger surface area and stability of the implanted pin.
Table 2: Comparison of component weights between the DREAM implant and the implant described by Vöröslakos et al.21. Please click here to download this Table.
Second, cementing of the microdrive should generally occur prior to the insertion of the probe in the brain. This prevents lateral movement of the probe inside the brain if the microdrive is not perfectly fixed in the stereotactic holder during insertion. To check the placement of the probe before cementing the microdrive in place, one can briefly lower the tip of the probe shank to ascertain where it will contact the brain since extrapolating the touchdown position can be difficult given the microscope's parallax shift. Once the microdrive position is established, one optionally can protect the craniotomy with silicone elastomer prior to cementing the microdrive to ensure that the cement does not accidentally make contact with the craniotomy; however, lowering the probe through the silicone elastomer is not recommended, as silicone elastomer residue can be pulled into the brain and cause inflammation and gliosis.
Third, depending on the experimental protocol used, a test recording directly after surgery may or may not be useful. Largely, neuronal activity recorded right after probe insertion will not be directly representative of activity recorded chronically, due to factors such as transient brain swelling and tissue movement around the probe, meaning that both insertion depth as well as spike waveforms are unlikely to stabilize directly. As such, immediate recordings can mainly serve to ascertain general signal quality and implant integrity. It is recommended that the moveable microdrive sled be utilized in subsequent days post-surgery once the brain has stabilized to fine-tune the position. This also helps to avoid moving the probe by more than 1000 µm per day, minimizing damage to the recording site and thus improving recording site longevity.
Finally, users may wish to adapt the system to record from more than one target location. As this system is modular, the user has a lot of leeway on how to assemble and place components in relation to each other (see above and Supplementary Figure 3 and Supplementary Figure 4). This includes modifications that would allow a horizontally extended shuttle to be mounted on the microdrive, allowing for multiple probes or large multi-shank probes to be implanted, as well as the implantation of multiple individual microdrives (see Supplementary Figure 3 and Supplementary Figure 4). Such modifications only require the use of an adapted crown ring, with an increased number of mounting zones for connectors/interface boards/headstages. However, the space limitations of this design are dictated by the animal model, in this case, the mouse, which makes stacking multiple probes onto one microdrive more attractive in terms of footprint than implanting several microdrives independently of each other. The microdrives used here can support stacked probes, and thus, the only real limitation is the number of headstages or connectors that can fit the space and weight constraints defined by the animal model. Spacers can also be used to further increase non-vertical mounting and insertion paths.
In conclusion, this protocol allows for inexpensive, lightweight, and importantly adjustable implantation of a probe, with the added benefit of a microdrive design that prioritizes probe recovery. This tackles the problems of the prohibitive cost of single-use probes, the high barrier of surgical and implantation skills, as well as the fact that commercial solutions for chronic implantation are often difficult to adapt to unique use cases. These issues pose a pain point to labs already using acute electrophysiology and a deterrent to those that do not yet undertake electrophysiology experiments. This system aims to facilitate the wider uptake of chronic electrophysiology research beyond these limitations.
Disclosures
TS, AN, and MNH are co-founders of 3Dneuro B.V., which manufactures the open-source microdrives and Faraday crowns used in this protocol. FB and PT are part of the scientific advisory board of 33Dneuro B.V.. FB and PT do not receive any financial compensation for this position.
Acknowledgements
This work was supported by the Dutch Research Council (NWO; Crossover Program 17619 "INTENSE", TS) and has received funding from the European Union's Seventh Framework Program (FP7/2007-2013) under grant agreement No. 600925 (Neuroseeker, TS, FB, PT), as well as from the Max Planck Society.
Materials
| Name | Company | Catalog Number | Comments |
| 0.05" Solder Tail Socket | Mill-Max | 853-93-100-10-001000 | |
| 1,1'-dioctadecyl-3,3,3',3'- Reagent tetramethylindocarbocyanine perchlorate ('DiI'; DiIC18(3)) | ThermoFisher | D282 | Lipophilic dye used for easier histological verification of the probe location |
| Adhesive Putty (Blu-Tack) | Bostik | 308590110 | Variations (e.g. by Pritt) should be available in your stationary store |
| Agar | Sigma Aldrich | A1296 | Make with saline for conductivity. |
| Amplifier (Miniamp-64) | Cambridge Neurotech | Miniature and implantable amplifier and digitiser. Alternative Implantable digitiser, or implantable Omnetics connector use possible. | |
| Analgesic Cream (EMLA Cream) | Aspen | 39699/0088 | Analgesic cream used for operative pain containing prilocaine, lidocaine. |
| Angled Spacer | 3DNeuro | Angled spacer for non-perpendicular drive mounting.. Open souce, also available at https://github.com/zero-noise-lab/dream-implant/ | |
| Blue light curing LED | B.A. International | 818223 | Curing light for primer polymerisation. 420-480 nm wavelength |
| Bone wax | SMI | Z046 | Wax to protect craniotomy and probe post surgery. |
| Buprenorphine | Elanco Europe LTD | 401513 | Injectable Buprenorphine solution (0.3 mg/mL) |
| Copper mesh | Dexmet | 3CU6-050FA | Copper mesh used to electrically and physically shield probe and craniotomy. |
| Cyanoacrylate glue (Loctite) | Loctite | 1363589 | Cyanoacrylate gel glue |
| Dental Cement (SuperBond C&B) | Sun Medical | K058E | Dental cement (SuperBond) |
| Depilation Cream (Veet) | Veet | 310000091434 | Hair removal cream for removal of hair around surgical site. |
| Enrofloxacin (Baytril) | Elanco Europe LTD | 00879/4117 | Injectable enrofloxacin solution (25 mg/mL) |
| Faraday crown | 3DNeuro | 3D printed implantable protective cage. Open souce, also available at https://github.com/zero-noise-lab/dream-implant/ | |
| Faraday ring | 3DNeuro | 3D printed implantable protective ring for faraday cage. Open souce, also available at https://github.com/zero-noise-lab/dream-implant/ | |
| Haemostatic Sponge | SMI | ZHG101010 | Absorbable gelatin haemostatic sponge |
| Heat Shrink Tubing | HellermannTyton | TA32-9/3 BK | Heat Shrink tubing for making soft tipped forceps |
| Iodine | Braunol | 9322507 | Aqueous povidone-iodine solution. |
| Metamizole (Novalgin) | Sanofi-Aventis Gmbh | 4527098 | Injectable Metamizole (500mg/mL) |
| Metamizole (Novalgin) | Sanofi-Aventis Gmbh | 1553758 | Metamizole solution |
| Microdrive (R2Drive) | 3DNeuro | Recoverable Metal micro drive with moveable shuttle. Open souce, also available at https://buzsakilab.github.io/ 3d_print_designs/ | |
| Mineral Oil | Sigma-Aldrich | M5310-100ML | Oil used as solvent to create craniotomy protection gel. |
| Non-Shedding Wipes (Kimtech) | Kimtech | 7552 | Non-shedding wipes |
| Primer | Bisco | B-7202P | Universal skull adhesive preventing moisture from deteriorating the cement and providing a solid base to build up cement onto. |
| R2Drive holder | 3DNeuro | Stereotactic attachment for mounting R2Drive. Open souce, also available at https://buzsakilab.github.io/ 3d_print_designs/ | |
| Self-adherent wrap | 3M | VB050 | Protective wrap for implant post surgery |
| Silicon probe (H2) | Cambridge Neurotech | Chronically implantable linear silicon probe with 32 channels. Alternative Probe use possible. | |
| Silicone Elastomer (Duragel) | Cambridge Neurotech | Silicone Elastomer | |
| Silicone Plaster (Kwikcast) | WPI | KWIK-CAST | |
| Silver conductive epoxy | MG Chemicals | 8331D-14G | Silver epoxy |
| Size 5 Dumont forceps | FSTools | 11251-10 | Small forceps for lifting bone flap. |
| Stainless steel wire, Teflon coated | Science Products GmBH | SS-3T | Ground wire |
| Stereotax (RWD) | RWD | 68803 | Stereotax for surgical procedures on mice. |
| Tergazyme | Alconox | 1304 | A possible enzymatic cleaner to clean probe |
| Two Part Fast setting Epoxy Resin | Gorilla | EP3 | Epoxy for permanent bonding of DREAM implant parts. |
| Vannas Spring Scissors Round Handle | FSTools | 15403-08 | 0.075mm straight tipped spring rebound veterinary scissors. |
| Veterinary Cyanoacrylate glue (Vetbond) | 3M | 70-0068-5256-3 | Veterinary cyanoacrylate glue |
References
- Epsztein, J., Brecht, M., Lee, A. K. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron. 70 (1), 109-120 (2011).
- Okun, M., et al. Diverse coupling of neurons to populations in sensory cortex. Nature. 521 (7553), 511-515 (2015).
- Jun, J. J., et al. Fully integrated silicon probes for high-density recording of neural activity. Nature. 551 (7679), 232-236 (2017).
- Znamenskiy, P., Kim, M. -. H., Muir, D. R., Iacaruso, M. F., Hofer, S. B., Mrsic-Flogel, T. D. Functional specificity of recurrent inhibition in visual cortex. Neuron. 112 (6), 991-1000.e8 (2024).
- Rowland, J. M., et al. Propagation of activity through the cortical hierarchy and perception are determined by neural variability. Nat Neurosci. 26 (9), 1584-1594 (2023).
- Roth, M. M., Dahmen, J. C., Muir, D. R., Imhof, F., Martini, F. J., Hofer, S. B. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat Neurosci. 19 (2), 299-307 (2016).
- Zong, W., et al. Large-scale two-photon calcium imaging in freely moving mice. Cell. 185 (7), 1240-1256.e30 (2022).
- Demas, J., et al. High-speed, cortex-wide volumetric recording of neuroactivity at cellular resolution using light beads microscopy. Nat Methods. 18 (9), 1103-1111 (2021).
- Buzsáki, G., Anastassiou, C. A., Koch, C. The origin of extracellular fields and currents - EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 13 (6), 407-420 (2012).
- Polikov, V. S., Tresco, P. A., Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 148 (1), 1-18 (2005).
- Savya, S. P., et al. In vivo spatiotemporal dynamics of astrocyte reactivity following neural electrode implantation. Biomaterials. 289, 121784 (2022).
- Perge, J. A., et al. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J Neural Eng. 10 (3), 036004 (2013).
- Pachitariu, M., Steinmetz, N., Kadir, S., Carandini, M., Harris, K. D. Kilosort: realtime spike-sorting for extracellular electrophysiology with hundreds of channels. bioRxiv. , (2016).
- Buzsáki, G. Large-scale recording of neuronal ensembles. Nat Neurosci. 7 (5), 446-451 (2004).
- Kloosterman, F., et al. Micro-drive array for chronic in vivo recording: Drive fabrication. J Vis Exp. (26), e1094 (2009).
- Jacobs, T., Darch, H., Holtzman, T., De Zeeuw, C. I., Romano, V. Standard operating protocol: Implantation of Cambrige NeuroTech chronic silicon probe and mini-amp-64 digital headstage in mice. Protocol Exchange. , (2023).
- Juavinett, A. L., Bekheet, G., Churchland, A. K. An adaptable, reusable, and light implant for chronic Neuropixels probes. bioRxiv. , (2024).
- Kozai, T. D. Y., Jaquins-Gerstl, A. S., Vazquez, A. L., Michael, A. C., Cui, X. T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem Neurosci. 6 (1), 48-67 (2015).
- Nguyen, D. P., et al. Micro-drive array for chronic in vivo recording: tetrode assembly. J Vis Exp. (26), 1098 (2009).
- Biran, R., Martin, D. C., Tresco, P. A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 195 (1), 115-126 (2005).
- Vöröslakos, M., Petersen, P. C., Vöröslakos, B., Buzsáki, G. Metal microdrive and head cap system for silicon probe recovery in freely moving rodent. eLife. (10), e65859 (2021).
- . IMEC Neuropixels 1.0 User Manual V1.0.8 Available from: https://www.neuropixels.org/_files/ugd/328966_ca209d53ffb346b3bf98be39b903efa9.pdf (2023)
- Baranauskas, G., et al. Carbon nanotube composite coating of neural microelectrodes preferentially improves the multiunit signal-to-noise ratio. J Neural Eng. 8 (6), 066013 (2011).
- Niell, C. M., Stryker, M. P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 65 (4), 472-479 (2010).
- Okun, M., Carandini, M., Harris, K. D. Long term recordings with immobile silicon probes in the mouse cortex. bioRxiv. , (2015).
- Chung, J., Sharif, F., Jung, D., Kim, S., Royer, S. Micro-drive and headgear for chronic implant and recovery of optoelectronic probes. Sci Rep. 7 (1), 2773 (2017).
- Bimbard, C., et al. An adaptable, reusable, and light implant for chronic Neuropixels probes. bioRxiv. , (2024).
- Jones, E. A. A. Chronic recoverable Neuropixels in mice. protocols.io. , (2023).
- . Neuronexus Products - dDrive Available from: https://www.neuronexus.com/products/accessories/microdrives/ddrive (2024)
- van Daal, R. J. J., et al. Implantation of Neuropixels probes for chronic recording of neuronal activity in freely behaving mice and rats. Nat Protoc. 16 (7), 3322-3347 (2021).
- Guo, Z. V., et al. Procedures for behavioral experiments in head-fixed mice. PLoS One. 9 (2), e88678 (2014).
- Groblewski, P. A., et al. A standardized head-fixation system for performing large-scale, in vivo physiological recordings in mice. J Neurosci Methods. 346, 108922 (2020).
- Vasilev, D., Raposo, I., Totah, N. K. Brightness illusions evoke pupil constriction preceded by a primary visual cortex response in rats. Cereb Cortex. 33 (12), 7952-7959 (2023).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved