A subscription to JoVE is required to view this content. Sign in or start your free trial.
Setting a Successful Sorting for Extracellular Vesicle Isolation
In This Article
Summary
This protocol provides a detailed description of sorting extracellular vesicles (EVs) released by mesenchymal stromal cells. In particular, it focuses on the instrument setting and the optimization of the sorting conditions. The goal is to sort extracellular vesicles while preserving their characteristics.
Abstract
Extracellular vesicles (EVs) released by mesenchymal stromal cells (MSCs) contain a set of microRNAs with regenerative and anti-inflammatory roles. Therefore, purified MSC-EVs are envisioned as a next-generation therapeutic option for a wide array of diseases. In this protocol, we report the strategy for successfully sorting EVs from the supernatant of adipose-derived MSCs (ASCs), often used in orthopedics regenerative medicine applications.
First, we described the sample preparation, focusing on EV isolation and labeling steps with carboxyfluorescein succinimidyl ester (CFSE) for fluorescence detection; subsequently, we detailed the sorting process, which constitutes the main part of the protocol.
In addition to the rules defined by MISEV 2023 and MIFlowCyt EV guidelines, we applied specific experimental conditions concerning nozzle size, frequency, and sheath pressure. Morphological parameters are established using beads of diameters selected to cover the theoretical range of EV size. After ASC-EVs sorting, we performed a purity check of the sorted fraction by re-analyzing it with the sorter and verifying the EV size distribution with the nanoparticle tracking analysis technique.
Due to the increasing importance of EVs, having a pure population to study and characterize is becoming crucial. Here, we demonstrate a winner strategy to set up sorting to achieve this goal.
Introduction
Extracellular Vesicles (EVs) are a heterogeneous group of membrane-structured vesicles released by almost all cells, delimited by a lipid bilayer, unable to replicate on their own1. They can be found in several biofluids such as blood plasma, serum, saliva, breast milk, urine, bronchial lavage fluid, amniotic fluid, cerebrospinal fluid, and malignant ascites2. One of the main functions of EVs is to transport various molecules, including nucleic acids, proteins, lipids, and carbohydrates, between a donor and a recipient cell. This can occur through various mechanisms, such as direct membrane fusion, receptor-ligand intera....
Protocol
The protocol here consists of four parts: (1) Sample preparation, (2) ASC-EVs characterization, (3) ASC-EVs sorting, and (4) Post sorting analysis. A schematic representing the workflow is shown in Figure 1.
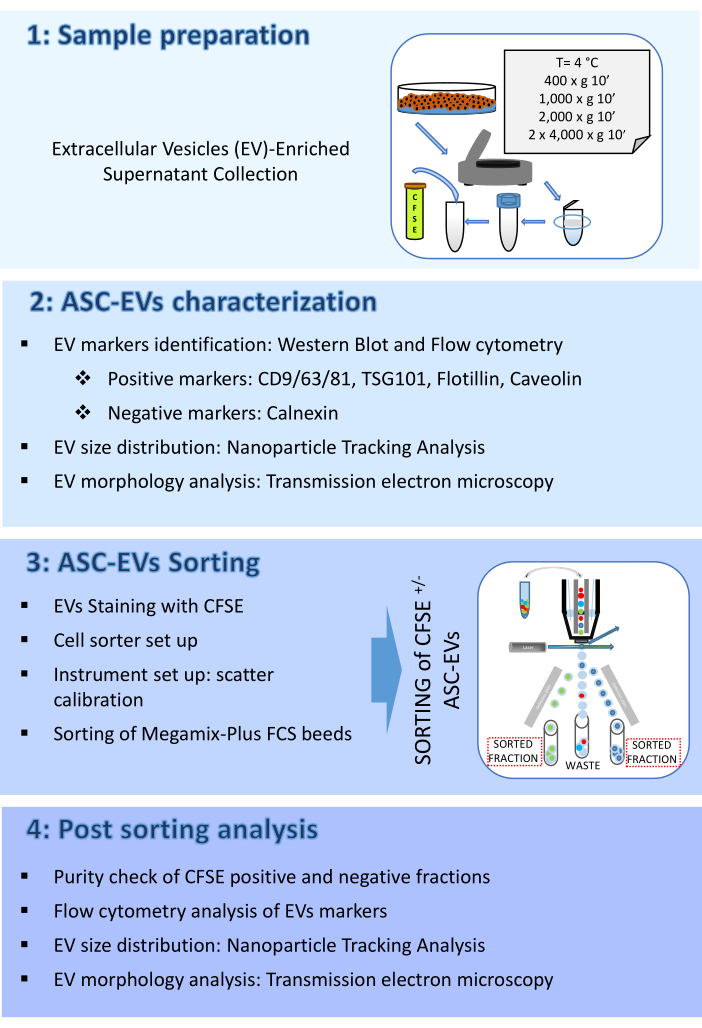
Figure 1: Protocol Flow chart. The flow chart shows the steps involved in t.......
Representative Results
The FSC polystyrene beads have been sorted to validate the instrument setup and sorting conditions. FSC polystyrene beads are a mix of fluorescent beads ranging from 100 nm, 300 nm, 500 nm, and 900 nm and are visible on the FITC channel. Figure 7A shows the SSC log scale versus the FITC log scale dot plot with the four populations of beads before sorting. The fluorescent populations of 100 nm, 300 nm, and 500 nm were gated and sorted. Sorted beads were analyzed for purity and enrichment as a.......
Discussion
Analyzing and sorting EVs is challenging due to their small size and the fact that they are near the detection limit of most flow cytometers. Our objective was to develop a protocol for isolating EVs derived from AMSCs labeled with CFSE. CFSE was selected as the staining method due to its reported high EVs labeling efficiency (≥90%), without the formation of unwanted particles such as protein aggregates given by antibodies. Nevertheless, it is possible that few EVs without esterase could be missed and future studie.......
Acknowledgements
We thank Emanuele Canonico for technical support. Part of this work was carried out in ALEMBIC, an advanced microscopy laboratory established by IRCCS Ospedale San Raffaele and Università Vita-Salute San Raffaele. The work of Enrico Ragni and Laura de Girolamo was supported by the Italian Ministry of Health, "Ricerca Corrente".
....Materials
| Name | Company | Catalog Number | Comments |
| 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester | Merck | 150347-59-4 | |
| Adipose Mesenchymal Stromal Cells | Wepredic, Parc d'affaires, 35760 Saint-Grégoire, France | Cells used in this study | |
| Alexa 488 anti-Caveolin | R&D Systems | IC5736G | Flow cytometry antibody |
| APC anti-human CD44 | BioLegend | 338805 | Flow cytometry antibody |
| APC anti-human CD63 | BioLegend | 353007 | Flow cytometry antibody |
| APC anti-human CD81 (TAPA-1) | BioLegend | 349509 | Flow cytometry antibody |
| APC anti-human CD9 | BioLegend | 312107 | Flow cytometry antibody |
| BC CytoFLEX S | Beckman Coulter | BC CytoFLEX S equipped with 3 lasers, Blue, Red and Violet | |
| Flow-Check Pro Fluorospheres | Beckman Coulter | A63493 | Fluorescent control beads for MoFLO Astrios EQ |
| FlowJo software (version 10.8.1) | BD | version 10.8.1 | Analysis software |
| IntraSure kit | BD Biosciences | 641776 | Fixation and permeabilization for intracellular staining |
| Megamix-Plus FSC | BioCytex | 7802 | FSC polystyrene beads |
| MoFLO Astrios EQ | Beckman Coulter | MoFLO Astrios EQ equipped with 4 lasers, Blue, Yellow - Green, Violet and Red | |
| Mouse anti-FLOT1 antibody | BD Transduction Laboratories | 610820 | Western Blot antibody |
| NanoSight NS300 | Malvern | NS300 | |
| Rabbit anti-Calnexin antibody | Origene | TA336279 | Western Blot antibody |
| Rabbit anti-CD9 and CD81 antibody (ExoAb antibody kit) | System Biosciences | EXOAB-KIT-1 | Western Blot antibodies |
| Rabbit anti-TSG101 antibody | Merck | HPA006161 | Western Blot antibody |
| Triton X-100 | Merck | 9036-19-5 | |
| Ultra Rainbow Fluorescent Particles | Spherotech | URFP-30-2 | |
| Ultracel 100 kDa MWCO | Merck | UFC910024 | |
| VER01 - Verity Shells | Exometry | Organo silica beads for scatter calibration |
References
- Welsh, J. A., et al. Minimal information for studies of extracellular vesicles (misev2023): From basic to advanced approaches. J Extracell Vesicles. 13 (2), e12404 (2024).
- Xu, R., Greening, D. W., Zhu, H. J., Takahashi, N., Simpson, R. J.
Explore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved