Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Un modelo de ratón tumoral con cámara de ventana de pliegue cutáneo dorsal para la microscopía intravital combinada y la resonancia magnética en la investigación traslacional del cáncer
* Estos autores han contribuido por igual
En este artículo
Resumen
La traslación de los hallazgos de la microscopía intravital se ve desafiada por su poca profundidad de penetración en el tejido. Aquí describimos un modelo de ratón de cámara de ventana dorsal que permite el registro conjunto de la microscopía intravital y las modalidades de imagen clínicamente aplicables (p. ej., TC, RM) para la correlación espacial directa, lo que podría agilizar la traducción clínica de los hallazgos de la microscopía intravital.
Resumen
Las imágenes intravitales preclínicas, como la microscopía y la tomografía de coherencia óptica, han demostrado ser herramientas valiosas en la investigación del cáncer para visualizar el microambiente tumoral y su respuesta al tratamiento. Estas modalidades de imagen tienen una resolución a escala micrométrica, pero tienen un uso limitado en la clínica debido a su poca profundidad de penetración en el tejido. Las modalidades de imagen más clínicamente aplicables, como la TC, la RMN y la PET, tienen una profundidad de penetración mucho mayor, pero tienen una resolución espacial (escala mm) comparativamente más baja.
Para trasladar los hallazgos de las imágenes intravitales preclínicas a la clínica, se deben desarrollar nuevos métodos para cerrar esta brecha de resolución entre lo micro y lo macro . Aquí describimos un modelo de ratón tumoral con cámara de ventana de pliegue cutáneo dorsal diseñado para permitir la obtención de imágenes preclínicas intravitales y clínicamente aplicables (TC y RM) en el mismo animal, y la plataforma de análisis de imágenes que vincula estos dos métodos de visualización dispares. Es importante destacar que el enfoque de cámara de ventana descrito permite que las diferentes modalidades de imagen se registren conjuntamente en 3D utilizando marcadores de referencia en la cámara de ventana para una concordancia espacial directa. Este modelo se puede utilizar para la validación de los métodos de imagen clínica existentes, así como para el desarrollo de otros nuevos a través de la correlación directa con los hallazgos intravitales de alta resolución "ground truth".
Por último, la respuesta tumoral a diversos tratamientos -quimioterapia, radioterapia, terapia fotodinámica- puede monitorizarse longitudinalmente con esta metodología utilizando modalidades de imagen preclínicas y clínicamente aplicables. Por lo tanto, el modelo de ratón tumoral con cámara de ventana de pliegue cutáneo dorsal y las plataformas de imágenes descritas aquí se pueden utilizar en una variedad de estudios de investigación sobre el cáncer, por ejemplo, para traducir los hallazgos de microscopía intravital preclínica a modalidades de imagen más aplicables clínicamente, como la TC o la RMN.
Introducción
La microvasculatura tumoral es un componente importante del microambiente tumoral que puede ser un objetivo para el tratamiento y un determinante de la respuesta al tratamiento. En el ámbito preclínico, la microvasculatura se estudia típicamente mediante microscopía intravital en modelos animales de cámara de ventana ortotópicos o heterotópicos 1,2. Esto tiene varias ventajas sobre los estudios histológicos, ya que las imágenes se realizan en tejidos vivos y el tumor puede ser monitoreado longitudinalmente durante varias semanas o incluso meses 2,3. Estos estudios pueden aprovechar las capacidades de imagen de alta resolución de la microscopía intravital para estudiar la administración de terapias al tumor 4,5, las causas de la resistencia al tratamiento6 y la respuesta de los microvasos a terapias como el tratamiento antiangiogénico 7,8 y la radioterapia 2,9.
La microscopía intravital desempeña claramente un papel importante en la investigación preclínica del cáncer; Sin embargo, ¿cómo se pueden medir las características microambientales del tumor en la clínica? La información microvascular sería útil en la clínica para medir el suministro de sangre y la hipoxia de las células tumorales, que es importante para determinar la resistencia al tratamiento en radioterapia10, así como la capacidad de la microvasculatura para administrar agentes quimioterapéuticos a las células tumorales circundantes11. Por ejemplo, en radioterapia, la información espacial sobre la estructura y función de la microvasculatura tumoral puede ayudar a personalizar el plan de tratamiento de un paciente ajustando el programa de fraccionamiento o aumentando preferentemente la dosis a las regiones avasculares y probablemente hipóxicas12.
La microscopía intravital puede medir estas importantes características microvasculares, ya que tiene una resolución muy alta (escala de μm); Sin embargo, su penetración profunda en el tejido está limitada a varios cientos de micras o unos pocos milímetros, lo que a lo sumo dificulta la implementación clínica. De hecho, existen algunas aplicaciones novedosas de la microscopía intravital en la clínica13; Sin embargo, todavía se limitan a exámenes de tejido a nivel de superficie como la piel14 o los revestimientos mucosos/endoteliales de varias cavidades corporales a través de catéteres/endoscopios flexibles15,16.
Más comúnmente, la microvasculatura se estudia utilizando modalidades de imagen como la TC17 o la RM18. Estas modalidades de imágenes clínicas pueden obtener imágenes a cualquier profundidad dentro del cuerpo, pero tienen una resolución espacial (escala milimétrica) mucho más baja. Por lo tanto, existe la necesidad de cerrar esta brecha de resolución entre la microscopía intravital preclínica y las modalidades de imagen clínica para llevar a la clínica información microvascular detallada y de alta resolución19. Se han desarrollado varios métodos de imagen funcional para mejorar las capacidades de imagen microvascular de las modalidades de imagen clínica, como la RM y la TC20 con contraste dinámico (DCE), y la RMN de movimiento incoherente intravoxel (IVIM)21. Sin embargo, estos son métodos basados en modelos que proporcionan mediciones indirectas de la microvasculatura y, por lo tanto, deben ser validados con mediciones apropiadas de la microvasculatura19,22.
Hemos desarrollado un modelo de ratón tumoral con cámara de ventana de pliegue cutáneo dorsal (DSFC) para cerrar esta brecha entre la microscopía intravital preclínica y las modalidades de imagen clínicamente aplicables, como la TC y la RM. El DSFC proporciona acceso directo al tumor para obtener imágenes de microscopía intravital de alta resolución a través de una ventana de vidrio, pero también imágenes clínicamente aplicables, como la resonancia magnética, ya que está hecho de materiales compatibles con la resonancia magnética (plástico y vidrio). Además, un código MATLAB incluido realiza el corregistro 3D multimodal para correlaciones espaciales directas entre la microscopía intravital preclínica y las modalidades de imagen clínicamente aplicables. Aquí describiremos el diseño y la cirugía para instalar el DSFC, así como el procedimiento para co-registrar la microscopía intravital y las modalidades de imagen clínicamente aplicables.
Protocolo
Todos los procedimientos con animales se realizaron de acuerdo con la Guía para el Cuidado y Uso de Animales de Experimentación, establecida por el Consejo Canadiense de Cuidado Animal. Los experimentos se realizaron de acuerdo con un protocolo aprobado por el Comité Institucional de Cuidado y Uso de Animales de la Red Universitaria de Salud en Toronto, Canadá.
1. Hito de la inoculación tumoral
NOTA: "Landmarking" se refiere al proceso de marcar la piel del ratón para indicar dónde se deben inyectar las células tumorales para optimizar la colocación de DSFC. Este procedimiento de marcación debe realizarse el mismo día o 1 día antes de la inoculación. Los inmunodeprimidos NOD. Para este trabajo se utilizó un ratón hembra Cg-Rag1tm1Mom Il2rgtm1Wjl/SzJ (NRG).
- Anestesiar al ratón utilizando isoflurano al 5% para la inducción y isoflurano al 2% para el mantenimiento (caudal de oxígeno ajustado a 0,5 L/min). Mantenga la temperatura corporal colocando el mouse sobre una almohadilla térmica envuelta en una alfombrilla quirúrgica esterilizada en autoclave.
- Prepare al ratón para la inoculación de células tumorales mediante el afeitado seguido de la aplicación de crema depilatoria médica. Retire completamente la crema depilatoria después de 30-60 s con una toalla de papel esterilizada húmeda. Aplique lubricante ocular veterinario para prevenir la sequedad.
- Desinfecte la piel con un hisopo con alcohol.
- Endereza suavemente el cuerpo del ratón y levanta la piel a lo largo de la columna vertebral del ratón. Con un marcador quirúrgico, dibuje un solo punto en un lado del ratón en el centro de la piel de la carpa. El punto debe estar ubicado aproximadamente en el centro de la columna torácica del ratón.
NOTA: Esta será la ubicación deseada del tumor.
2. Inoculación tumoral
NOTA: En este estudio, estamos utilizando una línea celular de cáncer de páncreas humano (BxPC3). También se pueden utilizar otras líneas celulares; Sin embargo, los pasos específicos del cultivo celular pueden variar en las diferentes líneas celulares. Consulte las instrucciones incluidas con las celdas para conocer las modificaciones del procedimiento a continuación.
- Cultivar las células a partir de 2 semanas antes de la inoculación utilizando un medio de crecimiento completo (medio Roswell Park Memorial Institute 1640 con 10% de suero fetal bovino y 1% de penicilina/estreptomicina) en un matraz de 75 ml a 37 °C y 5% de CO2. Asegúrese de que las células se mantengan en la fase de crecimiento exponencial.
NOTA: El número de paso debe restringirse a 10-15x y el matraz de 75 ml debe contener aproximadamente 6 millones de células (~70% de confluencia) en el momento de la inoculación. - Aspire el medio y lave las células con 5 mL de solución salina tamponada con fosfato (PBS) sin calcio ni magnesio.
- Añadir 5 mL de agente de disociación celular e incubar a 37 °C y 5% de CO2 durante 6-7 min. Confirme que las células se han desprendido con un microscopio y agregue 5 mL de medio de crecimiento completo.
NOTA: Golpear suavemente el costado del matraz puede ayudar a separar las celdas. - Transfiera la suspensión a un tubo de centrífuga de 15 ml y centrifugue a 500 × g durante 5 min para granular las celdas. Aspire el medio y vuelva a suspender las células en 5 mL de medio de crecimiento completo.
- Determine la concentración de células y el recuento total de células con un hemocitómetro.
- Centrifugar a 500 × g durante 5 min para pellets y aspirar el medio.
- Sobre la base del recuento total de células obtenido en el paso 2.5, añada la cantidad adecuada de medios de crecimiento completos para alcanzar una concentración en
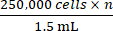 la que n es el número total de ratones que se van a inocular. Vuelva a suspender las células.
la que n es el número total de ratones que se van a inocular. Vuelva a suspender las células.
NOTA: El número objetivo de células a inocular por ratón es de 250.000. - Transfiera 1,5 mL que contiene 250.000 células × n a un tubo de microcentrífuga de 1,5 mL. Centrifugar el tubo de microcentrífuga de 1,5 ml a 500 × g durante 5 min y aspirar el medio.
NOTA: Cualquier exceso de suspensión celular se puede mantener en un baño de agua caliente calentado a 37 °C para ser utilizado para inoculaciones adicionales si es necesario. - Resuspender las células en 10 μL × n de PBS. Coloque las celdas sobre hielo para enfriar la suspensión.
NOTA: Una vez que las células se han enfriado en hielo, deben inyectarse en el ratón dentro de los 20 minutos. - Añadir 10 μL × n de membrana basal solubilizada con puntas de pipeta refrigeradas. Cargue agujas de insulina refrigeradas de calibre 29 con 20 μL cada una de las suspensiones celulares. Mantenga las jeringas en hielo.
- Anestesiar al ratón utilizando isoflurano al 5% para la inducción y isoflurano al 2% para el mantenimiento (caudal de oxígeno ajustado a 0,5 L/min).
- Coloque el mouse sobre una almohadilla térmica envuelta en una alfombrilla quirúrgica esterilizada.
- Desinfecte la piel en el lugar de la inyección con un hisopo con alcohol.
- Inserte la aguja ~ 1 cm antes del punto de referencia y mueva la aguja debajo de la piel hasta que esté en el punto de referencia. Coloque el lado biselado de la aguja hacia arriba e inyecte los 20 μL de suspensión celular.
- Espere 45 s para permitir que la membrana basal solubilizada se solidifique antes de retirar la jeringa.
- Retire al ratón de la anestesia, espere hasta que el ratón se vuelva ambulatorio y devuélvalo a su jaula con los otros ratones.
- Monitoree el tumor diariamente por palpación y deje que el tumor crezca durante 4 semanas o hasta que el tumor tenga 4-8 mm de diámetro. Aplicar la eutanasia al ratón si se cumple alguna de las siguientes condiciones: tamaño del tumor superior a 1,5 cm, tumor ulcerado o cualquier signo de enfermedad sistémica (letárgico, pérdida de peso superior al 20% del peso corporal normal, alteración de la deambulación, incapacidad para regular la temperatura corporal, anorexia, postura encorvada, signos visibles de dolor (expresiones faciales, etcétera) o deshidratación).
3. Cirugía de cámara de ventana
NOTA: La DSFC consta de cuatro piezas impresas en 3D, como se muestra en la Figura 1. Los esquemas de cada parte se incluyen en el Archivo Suplementario 1. Todas las piezas están impresas con una resina plástica transparente biocompatible. El conjunto de la cámara de la ventana principal consta de tres partes (Figura 1A-C) con un anillo marcador de referencia adicional (Figura 1D) que se puede colocar durante la resonancia magnética o la tomografía computarizada.
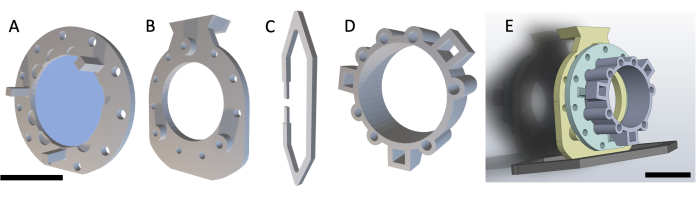
Figura 1: Esquema de la cámara de la ventana del pliegue cutáneo dorsal. La cámara de la ventana principal consta de tres partes. En primer lugar, (A) el marco frontal se sutura por debajo de la piel del ratón y contiene un cubreobjetos de vidrio fijado con pegamento curado con UV. (B) El marco trasero se sutura al marco delantero en el exterior de la piel. (C) El clip de soporte se fija a la parte inferior del marco trasero y mantiene el DSFC en posición vertical en el cuerpo del mouse. (D) El anillo marcador fiducial contiene siete "pozos" donde se pueden insertar marcadores fiduciales. El anillo marcador de referencia se puede fijar al marco delantero de la DSFC utilizando los tres postes de soporte. (E) Se muestra el conjunto DSFC completo con un anillo marcador de referencia. Barras de escala = 1 cm (A-D, en la parte inferior izquierda; E). Abreviatura: DSFC = cámara de ventana del pliegue cutáneo dorsal. Haga clic aquí para ver una versión más grande de esta figura.
- Administrar 1 mg/kg de buprenorfina de liberación sostenida 0,6 mg/ml por vía subcutánea 1-3 h antes de la cirugía. Asegúrese de que el ratón tenga el tamaño corporal y el peso suficientes para tolerar la DSFC sin constricción torácica.
- Esterilice las partes de la cámara de la ventana principal (Figura 1A-C) en un desinfectante líquido de alto nivel durante 12 minutos, seguido de remojo en alcohol isopropílico durante al menos 5 minutos.
- Anestesiar al ratón utilizando isoflurano al 5% para la inducción y isoflurano al 2% para el mantenimiento (caudal de oxígeno ajustado a 0,5 L/min).
- Transfiera el ratón a una alfombrilla quirúrgica estéril con una almohadilla térmica debajo.
- Afeita el ratón y aplica una crema depilatoria médica si hay vello. Después de 30-60 s, retire la crema depilatoria con toallas de papel húmedas estériles.
- Aplique lubricante ocular veterinario para prevenir la sequedad. Vuelva a aplicar cada 30 minutos o según sea necesario durante la cirugía.
- Desinfecta la piel aplicando un exfoliante de povidona yodada al 7,5% y aclarando con alcohol isopropílico al 70%. Deja que la piel se seque al aire.
- Aplique una solución de povidona yodada al 10% sobre la piel y deje que se seque al aire (Figura 2A).
- Reemplace el tapete quirúrgico por un nuevo tapete esterilizado.
- Asegúrese de que el ratón haya alcanzado el plano quirúrgico de la anestesia con un pellizco en el dedo del pie.
- Levanta la piel del ratón a lo largo de la columna vertebral y busca el tumor que crece en uno de los dos pliegues cutáneos laterales. Coloque el marco posterior de la DSFC (Figura 2B) en el mismo lado de la piel en el que está creciendo el tumor, asegurándose de centrar el tumor en el marco.
- Agregue tres suturas a cada uno de los tres orificios superiores de la DSFC y la guía quirúrgica para fijar la posición del marco trasero (Figura 2B).
- Inserte tres agujas a través del marco posterior de la DSFC en los orificios que contienen los espaciadores (Figura 2B).
NOTA: Hay tres "espaciadores" en el marco posterior de la DSFC para mantener un espacio entre los marcos delantero y trasero para garantizar que el flujo sanguíneo no se restrinja al tejido dentro de la DSFC. Los espaciadores son visibles en la Figura 1B. - Con un marcador quirúrgico, marque los seis puntos donde se ubicarán los espaciadores del marco trasero DSFC a ambos lados de la piel (puntos 1-6 en la Figura 2C, D).
NOTA: Las agujas insertadas deben usarse como guía para la ubicación precisa de estos puntos. - Dibuje un círculo de 1 cm de diámetro para indicar la piel que se eliminará en el lado opuesto al marco trasero (Figura 2D).
- Retire las agujas y el marco posterior de la piel cortando las suturas.
- Corte el orificio de 1 cm de diámetro en la piel que se marcó en el paso 3.15 con microtijeras quirúrgicas (Figura 2E).
- Coloque la sutura temporal para asegurar todas las partes de la cámara de la ventana a la piel. Inserte una sutura a través del punto 4, a través del orificio superior del marco frontal del DSFC y luego a través del punto 1 en el lado opuesto del mouse (Figura 2F y pasos 1-2 en la Figura Suplementaria S1).
- Pase la sutura a través del poste de soporte superior del marco trasero y luego vuelva a través del poste de soporte en el marco posterior que está más cerca de la cabeza del mouse (Figura 2F).
- Inserte la sutura a través del punto 2. A continuación, pase la sutura por el orificio correspondiente en el marco delantero (Figura 2G y paso 3 en la Figura Suplementaria S1).
- Desde el interior de la piel, pase la sutura a través del punto 6 como se indica en la Figura 2H y en los pasos 3-4 de la Figura Suplementaria S1.
- Pase la sutura a través del punto 5 como se indica en la Figura 2I y en el paso 4 de la Figura Suplementaria S1.
- Lleve la sutura a través del marco delantero, punto 3, y luego a través del marco trasero como se indica en la Figura 2J y el paso 5 en la Figura Suplementaria S1.
- Lleve la sutura de vuelta a través del punto 1, el marco frontal de la DSFC, y luego salga a través del punto 4 como se indica en la Figura 2K y el paso 6 en la Figura Suplementaria S1.
- Apriete todo el conjunto con esta sutura y deslice el marco delantero por debajo de la piel a través del orificio que se creó en el paso 3.17 (Figura 2L,M).
- Ata los dos extremos de esta sutura y corta el exceso de cuerda.
- Realice suturas permanentes a través de los orificios alrededor del perímetro de los marcos delantero y trasero de la DSFC. Sutura los dos marcos juntos en el patrón que se muestra en la Figura 2N y la Figura Suplementaria S2.
- Corta y retira la sutura temporal.
- Fije el clip de soporte al marco posterior de la DSFC deslizándolo en la protuberancia desde el marco posterior (Figura 2O).
NOTA: En la Figura 2P, Q, se muestra una imagen del ratón 2 semanas después de la cirugía. El clip de soporte se utiliza para mantener el DSFC en posición vertical en el mouse para reducir la tensión y las molestias de la piel. - Administrar 5 mg/kg de peso corporal Meloxicam, un fármaco antiinflamatorio no esteroideo, por vía subcutánea para reducir el dolor y la inflamación.
- Retire al ratón de la anestesia, espere hasta que el ratón se vuelva ambulatorio y devuélvalo a su jaula.
- Controlar el ratón 2-3 h después de la cirugía y luego diariamente durante al menos 1 semana. Considere el criterio de valoración alcanzado 2 meses después de la cirugía en cámara de ventana o si se cumple alguna de las condiciones del paso 2.17 (lo que ocurra primero).
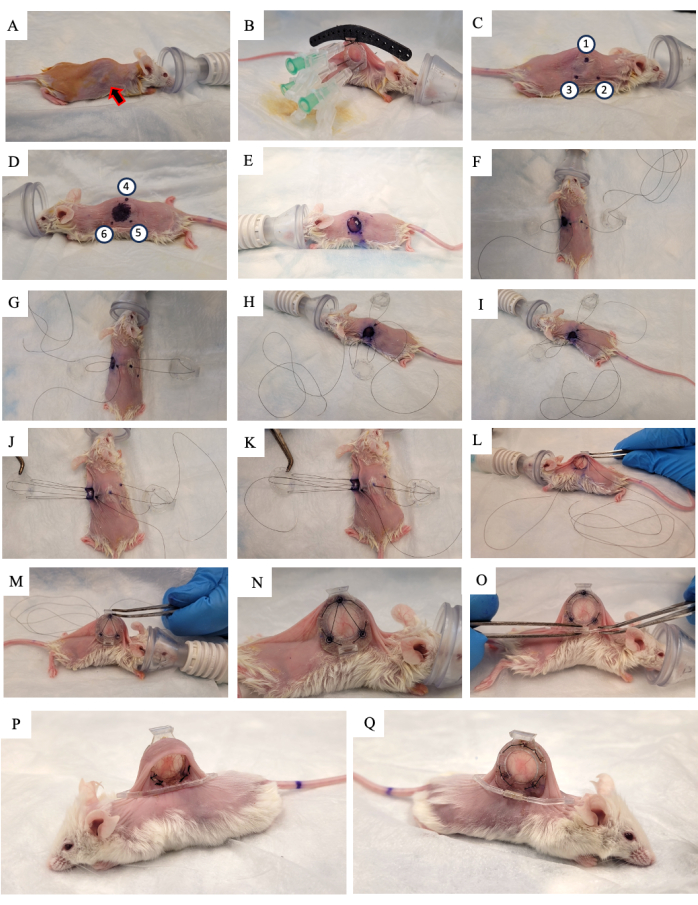
Figura 2: Procedimiento quirúrgico DSFC. (A) El ratón se prepara para la cirugía mediante la eliminación del vello y la desinfección de la piel. El tumor subcutáneo se indica con la flecha. (B) El marco trasero se coloca en la posición adecuada y se asegura con tres jeringas, así como con suturas temporales fijadas a la guía quirúrgica negra. (C,D) Las ubicaciones de los espaciadores (puntos 1-6) y el orificio están marcados a ambos lados de la piel. (E) Se retira la piel. (De F a K) Se enhebra una sutura temporal a través de las dos capas de piel, marcos delanteros y traseros de la DSFC para asegurar todas las partes juntas. (L,M) La sutura temporal se aprieta y el marco frontal se inserta debajo de la piel. (N) Se colocan ocho suturas permanentes para asegurar el DSFC. (O) Finalmente, se retira la sutura temporal y se coloca el clip de soporte. (P,Q) El mismo ratón se muestra 2 semanas después de la cirugía desde ambos lados. Abreviatura: DSFC = cámara de ventana del pliegue cutáneo dorsal. Haga clic aquí para ver una versión más grande de esta figura.
4. Imágenes ópticas
- Permita que el ratón sane y que la inflamación disminuya durante al menos 5 días después de la cirugía antes de la toma de imágenes.
- Anestesiar al ratón utilizando isoflurano al 5% para la inducción y isoflurano al 2% para el mantenimiento (caudal de oxígeno ajustado a 0,5 L/min).
- Aplique lubricante ocular veterinario para prevenir la sequedad. Vuelva a aplicar cada 30 minutos o según sea necesario.
- Asegure el ratón en una etapa de imagen con un accesorio de anestesia gaseosa como se muestra en la Figura Suplementaria S3.
- Obtenga una imagen de microscopía de campo claro de amplio campo visual (>1,5 cm de ancho). Asegúrese de que las hendiduras del marcador de referencia en el marco frontal de la DSFC sean visibles.
NOTA: Hay siete hendiduras alrededor del perímetro del vidrio en el marco frontal que se alinean con los siete pozos de marcadores fiduciales en el accesorio del anillo de marcador de referencia. Estas hendiduras son visibles en la Figura 1A. - El mismo día, obtener una imagen microvascular utilizando la modalidad de microscopía intravital de elección. Para ello, utilice la misma etapa de imagen del paso 4.4 (figura complementaria S3). Retire al ratón de la anestesia, espere hasta que el ratón se vuelva ambulatorio y devuélvalo a su jaula.
NOTA: Utilizamos la tomografía de coherencia óptica de varianza moteada (svOCT) para obtener imágenes microvasculares 3D de alta resolución.
5. Resonancia magnética
- Coloque la jaula del ratón debajo de una lámpara de calor para calentar a los ratones durante aproximadamente 15 minutos antes de anestesiar al ratón.
NOTA: El calentamiento promueve la vasodilatación, lo que ayudará en la colocación del catéter en la vena de cola. - Anestesiar al ratón utilizando isoflurano al 5% para la inducción y isoflurano al 2% para el mantenimiento (caudal de oxígeno ajustado a 0,5 L/min).
- Registre el peso del ratón mediante una báscula electrónica para la correcta dosificación del fármaco.
NOTA: Para obtener mediciones precisas del peso corporal, asegúrese de restar el peso del conjunto DSFC (0,83 g). - Coloque el ratón en la cama de resonancia magnética y aplique lubricante ocular veterinario para evitar la sequedad.
- Mantenga la temperatura corporal del ratón utilizando un calentador de agua y un sistema de bomba.
- Coloque una almohada de monitorización respiratoria debajo del diafragma del ratón y manténgala entre 30 ± 5 respiraciones/min.
- Si utiliza un agente de contraste, inserte una aguja de mariposa de 27 G en la vena de la cola con microtubos adjuntos (30 μL de volumen muerto precargados con solución salina de herapina al 1%). Asegure la aguja y el microtubo a la cama de resonancia magnética con cinta quirúrgica.
- Asegure la cámara de la ventana en un "dispositivo de inmovilización" impreso en 3D como se muestra en la Figura 3.
- Con una aguja de 18 G, inyecte lubricante ocular veterinario en los siete tubos del marcador fiducial de resonancia magnética.
- Fije el marcador de referencia a la DSFC alineando los tres conectores cuadrados con los tres postes que sobresalen del marco frontal (Figura 3).
- Conecte el catéter de la vena de cola a la línea de administración del medicamento y la jeringa en la bomba automática. Inserte la cama en un escáner de resonancia magnética preclínica de 7 teslas.
- Adquiera conjuntos de imágenes coronales y axiales ponderadas en T2 (T2w) para visualizar el plano de la cámara de la ventana (tiempo de eco TE = 25 ms; tiempo de repetición TR = 2.500 ms; campo de visión de 40 x 40 mm con una matriz de 64 x 64 para una resolución en el plano de 0,5 x 0,5 mm; grosor de corte de 0,5 mm; 25 s). Prescribir un conjunto sagital ponderado en T2 (TE = 25 ms; TR = 2.500 ms; Campo de visión de 32 x 32 mm con matriz de 128 x 128 para una resolución en el plano de 0,25 x 0,25 mm; al menos 11 cortes de imagen; espesor de corte de 0,5 mm; 87 s), que luego se rota en el plano de la DSFC y los marcadores fiduciales, según las vistas coronal y axial, como se muestra en la Figura 4. Reoriente y coloque iterativamente el conjunto sagital hasta que los cortes de imagen estén completamente alineados, de modo que el corte 5 contenga completamente la señal tisular en la DSFC y el corte 6 no contenga ninguna señal tisular en la DSFC.
- Realizar imágenes microvasculares utilizando el método de resonancia magnética microvascular de elección.
NOTA: Para las adquisiciones de RM microvascular, no es necesario obtener imágenes de los cortes de marcadores de referencia, ya que las imágenes se adquieren en el mismo marco de referencia de imágenes que las imágenes de registro T2w.- Adquiera todas las adquisiciones detalladas en los pasos 5.12 y 5.13 en un campo de visión de 32 x 32 mm con una matriz de 64 x 64 para una resolución en el plano de 0,5 x 0,5 mm. Para todas las adquisiciones de resonancia magnética, utilice pulsos de RF consistentes para mejorar la consistencia geométrica a través del plano (excitación sinc; reenfoque sinc3; ancho de banda de 2.484 Hz).
- Para la obtención de imágenes DCE:
- Si se desea medir la concentración de gadolinio, se obtienen mapas T1 utilizando imágenes 2D-RARE adquiridas en tiempos de repetición variables (TE = 7 ms; Factor de rareza = 2; TR = 350, 500, 750, 1.000, 1.500, 2.500 y 4.000 ms; 8 min 28 s).
- Realice imágenes de series temporales utilizando imágenes raras 2D (TE = 8,1 ms; Factor de rareza = 2; TR = 200 ms; ángulo de giro = 90°; resolución temporal = 12,8 s; 188 repeticiones; Tiempo total de monitoreo = 40 min 6 s).
- Inyecte 0,75 mmol/kg de peso corporal con Gadobutrol durante 10 s a través de la vena de cola después de completar cinco repeticiones de imágenes utilizando una bomba de jeringa automatizada compatible con RM.
- Para la resonancia magnética IVIM:
- Realice imágenes ponderadas en difusión con los siguientes valores B: 0, 20, 40, 60, 80, 100, 150, 200, 400, 600, 800, 1.000 s/mm2 con tres promedios de B = 0 s/mm2 y muestreo isotrópico (TE = 16 ms; TR = 800 ms; duración del gradiente = 2,2 ms; separación de gradiente = 9 ms; 61 min).
NOTA: Las imágenes ponderadas por difusión se adquieren utilizando una lectura de transformada de Fourier 2D, en lugar de con imágenes ecoplanares propensas a la distorsión, para garantizar la consistencia geométrica del tumor y las señales de tejido circundante dentro de la DSFC a través de los conjuntos de imágenes y con las imágenes de microscopía intravital.
- Realice imágenes ponderadas en difusión con los siguientes valores B: 0, 20, 40, 60, 80, 100, 150, 200, 400, 600, 800, 1.000 s/mm2 con tres promedios de B = 0 s/mm2 y muestreo isotrópico (TE = 16 ms; TR = 800 ms; duración del gradiente = 2,2 ms; separación de gradiente = 9 ms; 61 min).
- Retire al ratón de la anestesia, espere hasta que el ratón se vuelva ambulatorio y devuélvalo a su jaula.
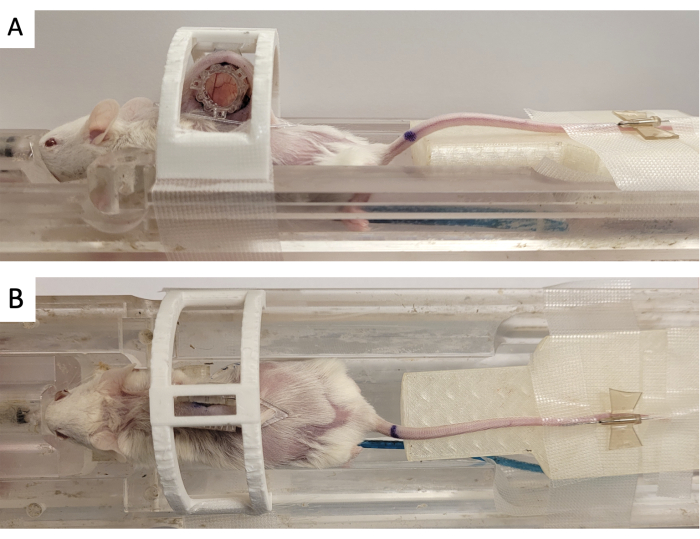
Figura 3: Configuración de las imágenes de resonancia magnética DSFC. (A) Vistas laterales y (B) superiores del ratón colocado en la cama de resonancia magnética con DSFC asegurado e inmovilizado. El ratón tiene un catéter de vena de cola para la inyección de agente de contraste y el anillo de fabricación fiducial está fijado al marco frontal del DSFC. Abreviaturas: DSFC = cámara de la ventana del pliegue cutáneo dorsal; RM = resonancia magnética. Haga clic aquí para ver una versión más grande de esta figura.
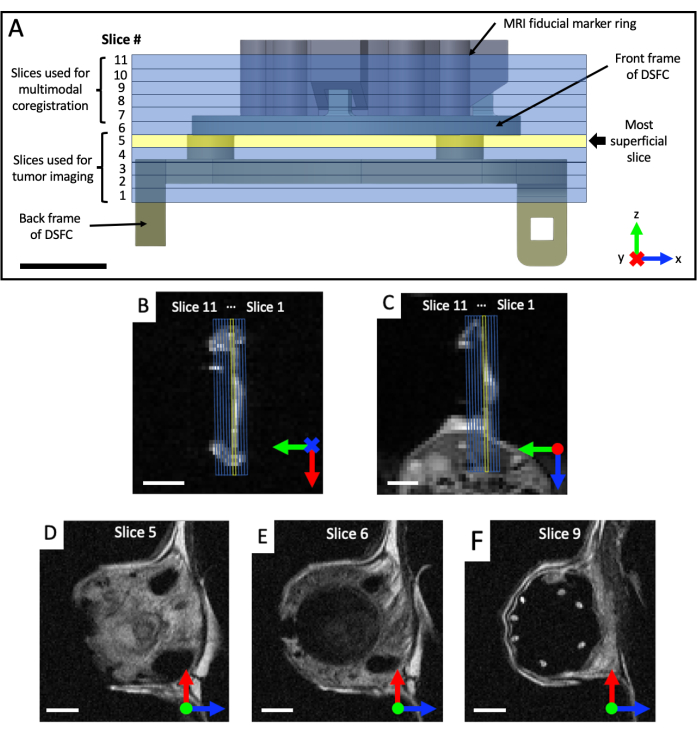
Figura 4: Ubicaciones de los cortes de RMN con respecto a los marcadores de referencia y la cámara de la ventana. (A) Un diagrama de la DSFC con la fijación del anillo marcador de referencia con los 11 cortes de RMN superpuestos. Se deben adquirir varias imágenes ponderadas en T2 para garantizar que los cortes estén correctamente alineados con la DSFC y el tejido. (B,C) Correcta colocación de las 11 lonchas con respecto al tejido en la DSFC desde diferentes orientaciones. (D) El segmento 5 es el segmento más superficial donde se realizará el análisis de correlación de intermodalidad. (E) La rebanada 6 no contiene ninguna señal tisular que indique que está correctamente alineada con la DSFC. (F) Finalmente, los 7 marcadores de referencia son claramente visibles en la porción 9. Barras de escala = 5 mm. Una 'X' en el eje indica que el eje va a entrar en la página y un círculo indica que el eje va a salir de la página. Abreviaturas: DSFC = cámara de la ventana del pliegue cutáneo dorsal; RMN = imágenes por resonancia magnética. Haga clic aquí para ver una versión más grande de esta figura.
6. Registro conjunto de resonancia magnética a microscopía intravital
- En MATLAB, abra el archivo Multimodal_Image_Register.m contenido en el archivo complementario 2.
- Al espacio de trabajo, cargue la imagen microvascular (Figura 5A), la imagen de microscopía de campo claro (Figura 5B) y los datos de resonancia magnética microvascular (mapas de parámetros de resonancia magnética IVIM y/o DCE).
- Haga clic en el botón Ejecutar .
- Usando el buscador de archivos emergentes, navegue hasta el archivo que contiene los cortes de resonancia magnética T2w.
- Seleccione hasta cuatro cortes de resonancia magnética T2w que muestren claramente los marcadores de referencia (Figura 5C).
- Se mostrará una interfaz de usuario que muestra la imagen de microscopía (Figura 5B) y los cortes de resonancia magnética T2w promediados en profundidad (Figura 5C). Coloque un punto en cada uno de los siete marcadores fiduciales en la imagen de resonancia magnética y hágalos coincidir con su punto correspondiente en la imagen de microscopía (hendiduras de marcadores de referencia alrededor del perímetro del vidrio en el marco frontal del DSFC) como lo indican los puntos verdes en la Figura 5B, C.
- Cierre la ventana.
- Aparecerá una figura que contiene las imágenes de resonancia magnética y microscopía superpuestas para ayudar a evaluar la calidad del registro entre los dos conjuntos de datos. Si el registro es adecuado, continúe el código escribiendo y y, a continuación, pulsando Intro en la ventana de comandos. De lo contrario, vuelva a intentar este paso escribiendo n en la ventana de comandos y pulsando Intro.
NOTA: El registro conjunto de datos de resonancia magnética exitoso con la imagen de microscopía significa que los siete marcadores fiduciales brillantes del conjunto de datos de resonancia magnética están centrados y completamente contenidos dentro de sus hendiduras correspondientes en el marco frontal de la DSFC. - Se mostrará una interfaz de usuario que muestra la imagen de microscopía (Figura 5B) y el conjunto de datos de imágenes microvasculares svOCT (Figura 5A). Seleccione al menos tres puntos de referencia microvascular en la imagen microvascular svOCT y los puntos correspondientes en la imagen de microscopía, como lo indican los puntos azules en la figura 5A,B.
- Cierre la ventana.
- Aparecerá una figura que contiene las imágenes superpuestas de svOCT y microscopía para ayudar a evaluar la calidad del corregistro entre los dos conjuntos de datos. Si el registro es adecuado, continúe el código escribiendo y y, a continuación, pulsando Intro en la ventana de comandos. De lo contrario, vuelva a intentar este paso escribiendo n en la ventana de comandos y pulsando Intro.
NOTA: El registro conjunto de datos svOCT exitoso con la imagen de microscopía significa que los recipientes de ambos conjuntos de datos están perfectamente superpuestos entre sí. - Cierre la ventana.
- A continuación, aparecerá la imagen de microcopia corregistrada. Contornea el tumor en esta imagen.
- Cierre la ventana.
- A continuación, se mostrarán varias figuras: el conjunto de datos microvasculares svOCT (Figura 5D), la imagen de microscopía co-registrada (Figura 5E) y los mapas de parámetros de RM co-registrados (Figura 5F). Guarde los mapas para su posterior análisis.
NOTA: Los mapas de resonancia magnética mostrados están restringidos al contorno tumoral dibujado por el usuario (Figura 5F).
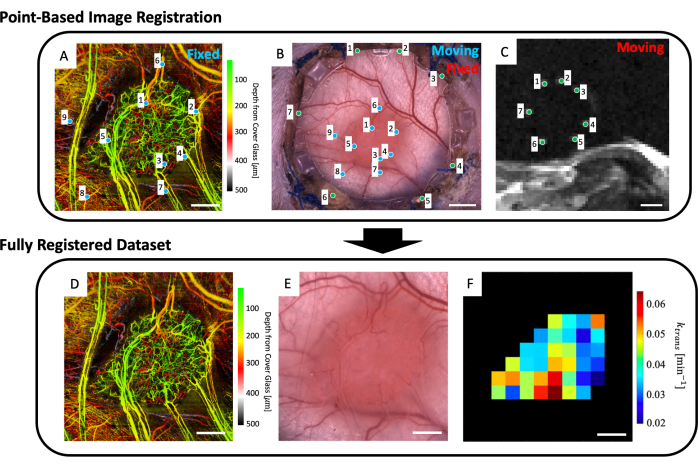
Figura 5: Corregistro multimodal basado en puntos. (A) Conjunto de datos de svOCT microvasculares codificados en profundidad en color; barra de escala = 1 mm. (B) Imagen de microscopía de campo claro de la cámara de la ventana; barra de escala = 2 mm. (C) Promedio de los cortes de resonancia magnética T2w 8-11 que muestran los siete marcadores fiduciales contenidos en el anillo marcador fiducial; barra de escala = 5 mm. (C) En primer lugar, el conjunto de datos de resonancia magnética T2w "en movimiento" se registra conjuntamente en la imagen de microscopía de campo claro "fija" utilizando los marcadores verdes introducidos por el usuario en ambos conjuntos de imágenes. A continuación, la imagen de microscopía de campo claro "en movimiento" y la imagen de resonancia magnética corregistrada se registran conjuntamente en el "conjunto de datos svOCT fijo" utilizando los marcadores azules de A y B. El conjunto de datos final registrado conjuntamente incluye el (D) svOCT, (E) la imagen de microscopía de campo claro y (F) el mapa de parámetros funcionales de resonancia magnética. Los vóxeles negros en F están fuera del tumor y, por lo tanto, no se tienen en cuenta en el análisis. Para D-F, barra de escala = 1 mm. Abreviaturas: svOCT = tomografía de coherencia óptica de varianza moteada; RMN = imágenes por resonancia magnética. Haga clic aquí para ver una versión más grande de esta figura.
Resultados
Se realizó una tomografía de coherencia óptica de varianza moteada (svOCT) para obtener imágenes microvasculares 3D de gran campo de visión (FOV) (6 x 6 mm,2 laterales x 1 mm de profundidad). Para la obtención de estas imágenes, se utilizó un sistema OCT de fuente de barrido previamente descrito, basado en un interferómetro de cuadratura23 23. Las imágenes de OCT se adquirieron uniendo dos escaneos FOV de 3 x 6 mm2 adyacentes lateralmente. Cada B-scan constó de 400...
Discusión
En este trabajo, hemos desarrollado un flujo de trabajo para realizar tanto microscopía intravital como imágenes clínicamente aplicables (TC, RM y PET) en el mismo animal. Esto se hizo con el objetivo de trasladar los hallazgos de la microscopía preclínica a la clínica mediante la correlación directa de la microscopía intravital con modalidades de imagen clínica como la resonancia magnética. Aunque los diseños convencionales de DSFC están hechos de metal 2,3
Divulgaciones
Los autores no tienen conflictos de intereses que revelar.
Agradecimientos
Agradecemos a la Dra. Carla Calçada (becaria postdoctoral, Princess Margaret Cancer Centre) y al Dr. Timothy Samuel (estudiante de doctorado, Princess Margaret Cancer Centre) por su ayuda con el cultivo de células tumorales y el desarrollo del protocolo de inoculación. La Dra. Kathleen Ma, la Dra. Anna Pietraszek y la Dra. Alyssa Goldstein (Centro de Investigación Animal, Centro Oncológico Princesa Margarita) ayudaron con el desarrollo del protocolo quirúrgico. Jacob Broske (tecnólogo de ingeniería médica, Princess Margaret Cancer Centre) y Wayne Keller (ejecutivo de clientes de hardware, Javelin Technologies, una empresa del grupo TriMech) imprimieron en 3D las cámaras de las ventanas. James Jonkman (Centro de Microscopía Óptica Avanzada, Red de Salud de la Universidad) proporcionó una valiosa orientación para la adquisición de imágenes de microscopía de campo claro y fluorescencia.
Materiales
| Name | Company | Catalog Number | Comments |
| Cell Culture Materials | |||
| BxPC-3 Human Pancreatic Cancer Cells | ATCC (American Type Culture Collection) | CRL-1687 | |
| Corning Matrigel Basement Membrane Matrix, LDEV-free, 10 mL | Corning | 354234 | |
| Corning Stripettor Ultra Pipet Controller | Corning | 07-202-350 | |
| Dulbecco Phospphate buffered saline without Calcium, Magnesium, or phenol red, 500 mL | Gibco | 14190144 | |
| Fetal Bovine Serum (Canada), 500 mL | Sigma-Aldrich | F1051-500ML | |
| Penicillin-Streptomycin 100x (liquid,stabilized, sterile-filtered, cell culture tested) | Sigma-Aldrich | P4333-100ML | |
| RPMI Medium 1640 (1x), liquid; with L-Glutamine, 500 mL | Gibco | 11875093 | |
| TrypLE Express Enzyme, 500 mL | Gibco | 12605028 | |
| Window Chamber Materials | |||
| 12 mm Glass Coverslip | Harvard Apparatus | CS-12R No. 1.5 | |
| Connex 500 3D Printer | Stratasys | N/A | |
| Biocompatible clear MED610 resin | Stratasys | RGD810 | |
| Loctite AA 3105 UV curable glue | Loctite | LCT1214249 | |
| Window chamber back frame | Trimech Inc | N/A | |
| Window chamber fiducial marker | Trimech Inc | N/A | |
| Window Chamber front frame | Trimech Inc | N/A | |
| Window chamber support clip | Trimech Inc | N/A | |
| inoculation and Surgery Materials | |||
| BD SafetyGlide Insulin Syringes with Permanently Attached Needles, 0.5 mL, 29 G x 1/2" | BD | CABD305932 | |
| Betadine Solution | Betadine | AP-B002C2R98U | |
| Cidex OPA 14 Day Solution 3.8 L | ASP | JOH20394 | |
| Disposable Surgical Underpads 23 inch x 24 inch | Kendall | 7134 | |
| Eye lubricant | Optixcare | 50-218-8442 | |
| Hair removal cream | Nair | 061700222611 | |
| Halstead Hemostatic Forceps | Almedic | 7742-A12-150 | |
| Heating pad | Sunbeam | B086MCN59R | |
| Iris Scissors | Almedic | 7601-A8-690 | |
| Isoflurane | Sigma | 792632 | |
| Metacam | Boehringer Ingelheim Animal Health USA Inc | NDC 0010-6015-03 | |
| NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl/SzJ mouse | the Jackson laboratory | 7799 | |
| Peanut Clipper & Trimmer | Wahl | 8655-200 | |
| SOFSILK Nonabsorbable Surgical Suture #5-0 with 3/8" Taper point needle (17 mm) (Wax Coated,Braided Black Silk, Sterile) | Syneture | VS880 | |
| Splinter Forceps | Almedic | 7725-A10-634 | |
| MR Imaging | |||
| 3D printed window chamber immobilization device. | custom 3D printed, refer to figure 3 for details. | ||
| Convection heating device | 3M Bair Hugger | 70200791401 | |
| Drug injection system | Harvard Apparatus | PY2 70-2131 | PHD 22/2200 MRI compatible Syringe Pump |
| Gadovist 1.0 | Bayer | 2241089 | |
| Respiratory monitoring system | SAII | Model 1030 | MR-compatible monitoring and gating system for small animals. |
| Tail vein catheter (27 G 0.5" ) | Terumo Medical Corp | 15253 | |
| Optical Imaging | |||
| 3D printed imaging stage | Custom 3D printed, refer to supplementary figure 3 for details. | ||
| 12 V 7 W Flexible Polyimide Heater Plate Thin Adhesive PI Heating Film 25 mm x 50 mm | BANRIA | B09X16XCVS | Heating element used for mouse body temeprature regulation. |
| DC power supply | BK Precission | 1761 | Used to power the heating element. |
| Leica MZ FLIII | Leica Microsystems | 15209 | |
| svOCT imaging system | In-house made imaging system. Details can be found in reference 23. | ||
| Software | |||
| MATLAB Software | MathWorks | R2020A |
Referencias
- Fukumura, D., Duda, D. G., Munn, L. L., Jain, R. K. Tumor microvasculature and microenvironment: Novel insights through intravital imaging in pre-clinical models. Microcirculation. 17 (3), 206-225 (2010).
- Demidov, V., et al. Preclinical longitudinal imaging of tumor microvascular radiobiological response with functional optical coherence tomography. Sci Rep. 8 (1), 38 (2018).
- Alieva, M., Ritsma, L., Giedt, R. J., Weissleder, R., van Rheenen, J. Imaging windows for long-term intravital imaging. IntraVital. 3 (2), e29917 (2014).
- Dreher, M. R., et al. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 98 (5), 335-344 (2006).
- Momiyama, M., et al. Subcellular real-time imaging of the efficacy of temozolomide on cancer cells in the brain of live mice. Anticancer Res. 33 (1), 103-106 (2013).
- Dadgar, S., Rajaram, N. Optical imaging approaches to investigating radiation resistance. Front Oncol. 9, 1152 (2019).
- Fukumura, D., Jain, R. K. Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization. Microvasc Res. 74 (2-3), 72-84 (2007).
- Dirkx, A. E. M., et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 20 (6), 621-630 (2006).
- Allam, N., et al. Longitudinal in-vivo quantification of tumour microvascular heterogeneity by optical coherence angiography in pre-clinical radiation therapy. Sci Rep. 12, 6140 (2022).
- Stadlbauer, A., et al. Tissue hypoxia and alterations in microvascular architecture predict glioblastoma recurrence in humans. Clin Cancer Res. 27 (6), 1641-1649 (2021).
- Danquah, M. K., Zhang, X. A., Mahato, R. I. Extravasation of polymeric nanomedicines across tumor vasculature. Adv Drug Deliv Rev. 63 (8), 623-639 (2011).
- Bentzen, S. M., Gregoire, V. Molecular imaging-based dose painting: a novel paradigm for radiation therapy prescription. Semin Radiat Oncol. 21 (2), 101-110 (2011).
- Gabriel, E. M., Fisher, D. T., Evans, S., Takabe, K., Skitzki, J. J. Intravital microscopy in the study of the tumor microenvironment: from bench to human application. Oncotarget. 9 (28), 20165-20178 (2018).
- Demidov, V., et al. Preclinical quantitative in-vivo assessment of skin tissue vascularity in radiation-induced fibrosis with optical coherence tomography. J Biomed Opt. 23 (10), 1-9 (2018).
- Wallace, M. B., et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 31 (5), 548-552 (2010).
- Standish, B. A., et al. In vivo endoscopic multi-beam optical coherence tomography. Phys Med Biol. 55 (3), 615-622 (2010).
- Wang, J. H., et al. Dynamic CT evaluation of tumor vascularity in renal cell carcinoma. AJR Am J Roentgenol. 186 (5), 1423-1430 (2006).
- Tropres, I., et al. Imaging the microvessel caliber and density: Principles and applications of microvascular MRI. Magn Reson Med. 73 (1), 325-341 (2014).
- McDonald, D. M., Choyke, P. L. Imaging of angiogenesis: from microscope to clinic. Nat Med. 9, 713-725 (2003).
- O'Connor, J. P. B., et al. Dynamic contrast-enhanced imaging techniques: CT and MRI. Brit J Radiol. 84, S112-S120 (2011).
- Lima, M., Le Bihan, D. Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology. 278 (1), 13-32 (2015).
- Zabel, W. J., et al. Bridging the macro to micro resolution gap with angiographic optical coherence tomography and dynamic contrast enhanced MRI. Sci Rep. 12 (1), 3159 (2022).
- Mao, Y., Flueraru, C., Chang, S., Popescu, D. P., Sowa, M. G. High-quality tissue imaging using a catheter-based swept-source optical coherence tomography systems with an integrated semiconductor optical amplifier. IEEE Trans Instrum Meas. 60 (10), 3376-3383 (2011).
- Mariampillai, A., et al. Optimized speckle variance OCT imaging of microvasculature. Opt Lett. 35 (8), 1257-1259 (2010).
- Tofts, P. S., et al. Estimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusible tracer: standardized quantities and symbols. J Magn Res Imaging. 10 (3), 223-232 (1999).
- Khalifa, F., et al. Models and methods for analyzing DCE-MRI: a review. Med Phys. 41 (12), 124301 (2014).
- Reitan, N. K., Thuen, M., Goa, P. E., de Lange Davies, C. Characterization of tumor microvascular structure and permeability: comparison between magnetic resonance imaging and intravital confocal imaging. J Biomed Opt. 15 (3), 036004 (2010).
- Dhani, N. C., et al. Analysis of the intra- and intertumoral heterogeneity of hypoxia in pancreatic cancer patients receiving the nitroimidazole tracer pimonidazole. Br J Cancer. 113 (6), 864-871 (2015).
- Gaustad, J. V., Brurberg, K. G., Simonsen, T. G., Mollatt, C. S., Rofstad, E. K. Tumor vascularity assessed by magnetic resonance imaging and intravital microscopy imaging. Neoplasia. 10 (4), 354-362 (2008).
- Rouffiac, V., et al. Multimodal imaging for tumour characterization from micro to macroscopic level using a newly developed dorsal chamber designed for long-term follow-up. J Biophotonics. 13 (1), 201900217 (2020).
- Leung, H. M., Schafer, R., Pagel, M. M., Robey, I. F., Gmitro, A. F. Multimodality pH imaging in a mouse dorsal skin fold window chamber model. Proc SPIE Int Soc Opt Eng. 8574, 85740L (2013).
- Erten, A., et al. Magnetic resonance and fluorescence imaging of doxorubicin-loaded nanoparticles using a novel in vivo model. Nanomed. 6 (6), 797-807 (2010).
- Maeda, A., DaCosta, R. S. Optimization of the dorsal skinfold window chamber model and multi-parametric characterization of tumor-associated vasculature. Intravital. 3 (1), e27935 (2014).
- Allam, N., Taylor, E., Vitkin, I. A. Low-cost 3D-printed tools towards robust longitudinal multi-modal pre-clinical imaging. bioRxiv. , (2023).
- Alexander, S., Weigelin, B., Winkler, F., Friedl, P. Preclinical intravital microscopy of the tumour-stroma interface: invasion, metastasis, and therapy response. Curr Opin Cell Biol. 25 (5), 659-671 (2013).
- Steven, A. J., Zhuo, J., Melhem, E. R. Diffusion kurtosis imaging: an emerging technique for evaluating the microstructural environment of the brain.Am. J Roentgenol. 202 (1), W26-W33 (2014).
- Mayer, P., et al. Diffusion kurtosis imaging-a superior approach to assess tumor-stroma ratio in pancreatic ductal adenocarcinoma. Cancers (Basel). 12 (6), 1656 (2020).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados