The physical form of a substance changes on changing its temperature. For example, raising the temperature of a liquid causes the liquid to vaporize (convert into vapor). The process is called vaporization—a surface phenomenon. Vaporization occurs when the thermal motion of the molecules overcome the intermolecular forces, and the molecules (at the surface) escape into the gaseous state. When a liquid vaporizes in a closed container, gas molecules cannot escape. As these gas phase molecules move randomly about, they will occasionally collide with the surface of the condensed phase, and in some cases, these collisions will result in the molecules re-entering the condensed phase. The change from the gas phase to the liquid is called condensation.
Vaporization is an endothermic process. The cooling effect is evident after a swim or a shower. When the water on the skin evaporates, it removes heat from the skin and cools the skin. The energy change associated with the vaporization process is the enthalpy of vaporization, ΔHvap. For example, the vaporization of water at standard temperature is represented by:

The reverse of an endothermic process is exothermic. And so, the condensation of a gas releases heat:
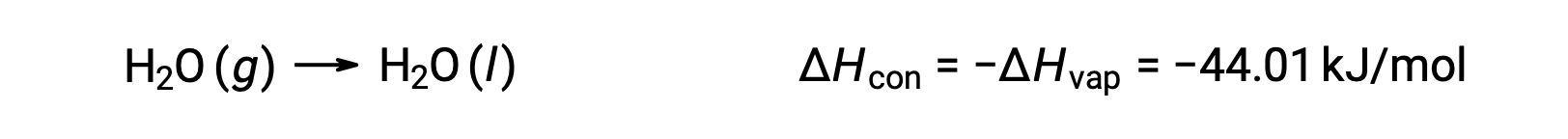
Vaporization and condensation are opposing processes; consequently, their enthalpy values are identical with opposite signs. While the enthalpy of vaporization is positive, the enthalpy of condensation is negative.
Different substances vaporize to different extents (depending on the strengths of their IMFs) and hence display different enthalpy of vaporization values. Relatively strong intermolecular attractive forces between molecules result in higher enthalpy of vaporization values. Weak intermolecular attractions present less of a barrier to vaporization, yielding relatively low values of enthalpies of vaporization.
This text is adapted from Openstax, Chemistry 2e, Section 10.3: Phase Transitions.
From Chapter 11:

Now Playing
11.7 : מעברי פאזה: אידוי ועיביוי
Liquids, Solids, and Intermolecular Forces
17.0K Views

11.1 : השוואה מולקולרית של גזים, נוזלים ומוצקים
Liquids, Solids, and Intermolecular Forces
40.1K Views

11.2 : כוחות בין מולקולריים לעומת תוך מולקולריים
Liquids, Solids, and Intermolecular Forces
84.6K Views

11.3 : כוחות בין מולקולריים
Liquids, Solids, and Intermolecular Forces
56.0K Views

11.4 : השוואת כוחות בין מולקולריים: נקודת התכה, נקודת רתיחה ומסיסות
Liquids, Solids, and Intermolecular Forces
43.5K Views

11.5 : מתח פנים, פעילות קפילרית וצמיגות
Liquids, Solids, and Intermolecular Forces
27.2K Views

11.6 : מעברי פאזה
Liquids, Solids, and Intermolecular Forces
18.5K Views

11.8 : לחץ אדים
Liquids, Solids, and Intermolecular Forces
33.9K Views

11.9 : משוואת קלאוזיוס- קלפרון
Liquids, Solids, and Intermolecular Forces
55.0K Views

11.10 : מעברי פאזה: התכה וקיפאון
Liquids, Solids, and Intermolecular Forces
12.2K Views

11.11 : מעברי פאזה: המראה וריבוץ
Liquids, Solids, and Intermolecular Forces
16.5K Views

11.12 : עקומות חימום וקירור
Liquids, Solids, and Intermolecular Forces
22.2K Views

11.13 : דיאגרמת פאזות
Liquids, Solids, and Intermolecular Forces
38.8K Views

11.14 : מבנים של מוצקים
Liquids, Solids, and Intermolecular Forces
13.5K Views

11.15 : מוצקים מולקולריים ויוניים
Liquids, Solids, and Intermolecular Forces
16.5K Views
See More
Copyright © 2025 MyJoVE Corporation. All rights reserved