Characterizing Mediated Extracellular Electron Transfer in Lactic Acid Bacteria with a Three-Electrode, Two-Chamber Bioelectrochemical System
In This Article
Summary
Here we present a protocol for characterizing mediated extracellular electron transfer (EET) in lactic acid bacteria using a three-electrode, two-chamber bioelectrochemical system. We illustrate this method with Lactiplantibacillus plantarum and the redox mediator 1,4-dihydroxy-2-naphthoic acid and provide a thorough description of the electrochemical techniques used to evaluate mediated EET.
Abstract
Many bacteria perform extracellular electron transfer (EET), whereby electrons are transferred from the cell to an extracellular terminal electron acceptor. This electron acceptor can be an electrode and electrons can be delivered indirectly via a redox-active mediator molecule. Here, we present a protocol to study mediated EET in Lactiplantibacillus plantarum, a probiotic lactic acid bacterium widely used in the food industry, using a bioelectrochemical system. We detail how to assemble a three-electrode, two-chambered bioelectrochemical system and provide guidance on characterizing EET in the presence of a soluble mediator using chronoamperometry and cyclic voltammetry techniques. We use representative data from 1,4-dihydroxy-2-naphthoic acid (DHNA)-mediated EET experiments with L. plantarum to demonstrate data analysis and interpretation. The techniques described in this protocol can open new opportunities for electro-fermentation and bioelectrocatalysis. Recent applications of this electrochemical technique with L. plantarum demonstrated an acceleration of metabolic flux towards producing fermentation end-products, which are critical flavor components in food fermentation. As such, this system has the potential to be further developed to alter flavors in food production or produce valuable chemicals.
Introduction
Bioelectrochemical systems interface microbes with electrodes, allowing investigation of extracellular electron transfer (EET) mechanisms and providing renewable approaches to bioelectrocatalysis1,2,3. Microbes that naturally perform EET are known as exoelectrogens, which transfer electrons derived from metabolism to extracellular terminal electron acceptors, e.g., iron (hydr)oxides and electrodes1. First characterized in Geobacter and Shewanella species4,5, EET pathways have since been identified in many bacteria. These exoelectrogens play a central role in several microbial electrochemical technologies, such as generating electrical energy from waste streams, fixing CO2, and producing valuable chemicals via electrosynthesis1,6,7,8,9,10,11,12.
One such exoelectrogen is Lactiplantibacillus plantarum, a gram-positive lactic acid bacterium13. L. plantarum is a nomadic, probiotic bacterium that resides in a wide range of environments, including the guts of humans and other vertebrates, as well as many types of food such as meat, cereals, vegetables, and fermented foods and drinks14,15,16,17. Its genome encodes a flexible, heterofermentative metabolism, allowing successful adaptation across these diverse environments. It is well-studied, widely used in the food and health industries, and generally recognized as safe by the Food and Drug Administration18,19. As such, L. plantarum has the potential to serve as a useful platform for EET-based technologies.
Recent research in L. plantarum has identified a multi-gene operon encoding a complex EET pathway originally characterized in Listeria monocytogenes13,20. In L. plantarum, the proteins synthesized from this operon facilitate EET in a bioelectrochemical system (BES) when provided the quinone 1,4-dihydroxy-2-naphthoic acid (DHNA) as an electron mediator13. The first essential protein in this pathway is a membrane-bound NADH-quinone oxidoreductase (Ndh2), which oxidizes NADH and reduces DHNA. DHNA either delivers electrons directly to an electrode or indirectly via the accessory protein PplA (Figure 1)13,21,22. Recent research indicates L. plantarum may also use other quinones that are structurally similar to DHNA as electron mediators; however, L. plantarum is incapable of producing DHNA or these alternative quinones, thus mediators must be exogenously present in the environment for EET to occur13,22,23.
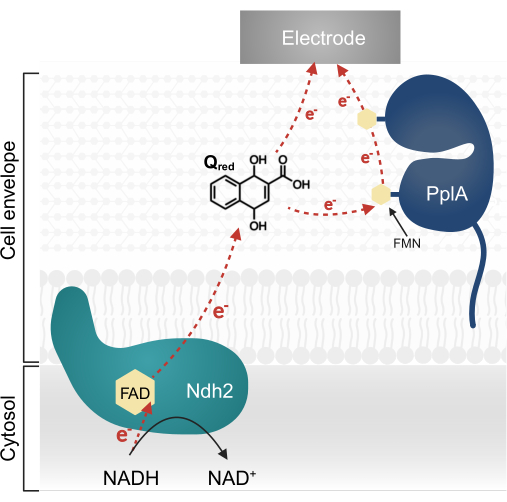
Figure 1: Electron flow in Lactiplantibacillus plantarum EET. Ndh2 passes electrons from NADH to the quinone DHNA. Electrons are shuttled to the electrode to produce current, either directly by reduced quinone or indirectly through the accessory protein PplA. Abbreviations: FAD = Flavin adenine dinucleotide; FMN = Flavin mononucleotide; EET = extracellular electron transfer; NADH = reduced nicotinamide adenine dinucleotide; Ndh2 = NADH-quinone oxidoreductase; DHNA = 1,4-dihydroxy-2-naphthoic acid; PplA = phospholipase A. Please click here to view a larger version of this figure.
In this article, we provide a comprehensive protocol for using a BES-based method to characterize DHNA-mediated EET in L. plantarum. A three-electrode, two-chamber system confines bacteria to the working electrode, allowing precise control of the potential applied to the bacteria while preventing crosstalk between the working and counter electrode. We present a comprehensive protocol spanning 5 days, which covers pre experiment preparation, BES assembly, EET analysis utilizing chronoamperometry (CA) and cyclic voltammetry (CV), and post experiment sample analysis. This protocol can be applied to unravel the mechanisms of EET pathways and to build systems for electrofermentation and electrocatalysis.
Protocol
NOTE: Two-chambered BES assemblies will be referred to as "reactors" in the following protocol.
1. Media preparation
- Prepare L. plantarum culturing media.
- Prepare commercial MRS (de Man Rogosa Sharpe) media as instructed and mMRS media24 as described in Table 1. Adjust the pH of mMRS to 6.5. Filter-sterilize both media by passing them through a 0.22 µm filter and store both at 4 ˚C until use.
- Prepare a Chemically Defined Medium with mannitol (mCDM)13,25 as described in Table 2, and adjust the pH to 6.5. Prepare enough medium to fill the anodic chamber of each reactor with 110 mL of medium. Filter-sterilize mCDM through a 0.22 µm filter.
NOTE: mCDM should be prepared fresh on the day of intended use. The component solutions may be prepared in advance. Filter-sterilize all component solutions through a 0.22 µm filter and store at 4 ˚C. When preparing Wolfe's vitamins, adjust the pH to 11 before sterilizing and store in darkness or wrapped in foil. When preparing Wolfe's minerals, adjust the pH to 8 after adding nitrilotriacetic acid (NTA). Then, add the remaining components, sterilize, and store in darkness or wrapped in foil.
- Prepare 1x PBS and autoclave to sterilize. Store at 4 ˚C for use in this protocol.
- Prepare commercial M9 media as instructed (Table 3) and autoclave to sterilize. Prepare enough media to fill the cathodic chamber of each reactor with 110 mL. Store at room temperature.
2. Day 1: BES reactor assembly and initial L. plantarum culturing
NOTE: Reference Figure 2 for a schematic of a BES reactor and a diagram detailing the assembly pieces indicated in the protocol.
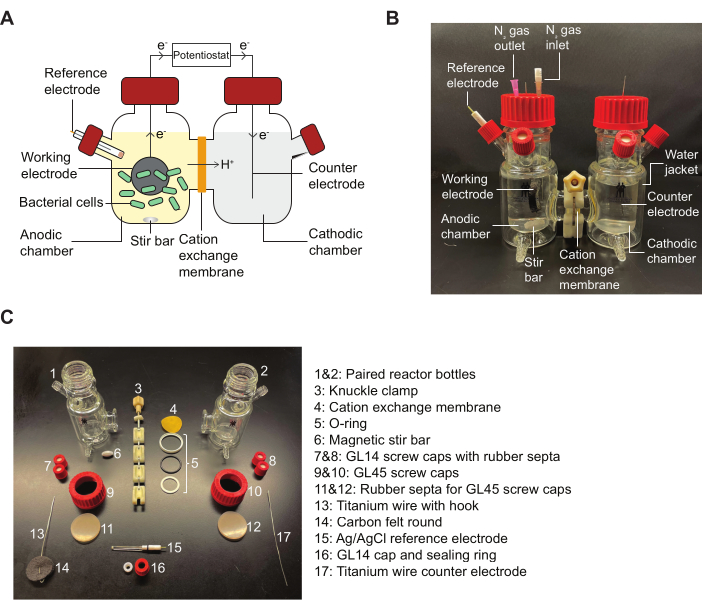
Figure 2: BES components and diagram for assembly. (A) A schematic of a two-chamber BES reactor. Bacteria (green) in the anodic chamber transfer electrons to a working electrode (black circle) in the presence of a quinone mediator. Electrons flow through the circuit to the cathodic chamber, allowing current measurements to be taken between the anode and cathode by a potentiostat. (B) An image depicting a fully assembled BES reactor, including N2 inlet and outlet needles in the anodic chamber. (C) An image depicting all parts of a disassembled reactor. Abbreviation: BES = bioelectrochemical system. Please click here to view a larger version of this figure.
- Assemble and sterilize reactors.
- Prepare the working and counter electrodes. Sand precut 1.0 mm diameter titanium wires for the working and counter electrodes with aluminum oxide sandpaper until evenly shiny. Using pliers, bend one end of each working electrode wire into a small hook. Slide a 16 cm2 carbon felt round onto each working electrode wire, weaving the wire in and out of the carbon felt round once and pulling the round down the wire until secured on the hook. Secure the working and counter electrodes into GL45 caps by piercing the rubber septum with the wire and pulling it through a few centimeters.
- To assemble paired reactors, first assemble the O-ring and place a precut cation exchange membrane that has been presoaked in water into the assembled O-ring. Place the O-ring with the membrane between the large bottom openings of two paired reactor bottles. Secure the reactor pair bottles and O-ring with the membrane with a knuckle clamp to finger tight. Drop a magnetic stir bar into each anodic chamber, and then close all small openings at the top of each bottle with GL14 caps fitted with rubber septa.
NOTE: Do not overtighten the knuckle clamps or any caps as this can lead to cracks or breaks in the bottles. - Fill each reactor bottle with 110 mL of deionized (DI) water, then close each bottle with the appropriate electrode-fitted GL45 cap. Insert caps fitted with a carbon felt round working electrode by gently pressing down the top of the felt round so the round remains on the hook.
NOTE: Fresh carbon felt is hydrophobic and repels water in the reactor in the initial assembly. Autoclaving will cause the carbon felt to become appropriately hydrophilic for experimentation. - Gather GL14 electrode caps fitted with silicone sealing rings.
- Gently loosen all GL45 caps prior to autoclaving. Autoclave water-filled reactors and electrode caps to sterilize. After autoclaving, allow the reactors to cool to room temperature.
- Culture L. plantarum NCIMB8826. Under sterile conditions, scrape some bacteria from the top of a glycerol stock and inoculate into 3 mL of commercial MRS media. Grow the culture overnight at 37 ˚C without shaking.
NOTE: L. plantarum does not use oxygen as a terminal electron acceptor; therefore, it is not necessary to oxygenate the media by shaking26. However, culturing conditions will vary if other microbes are used.
3. Day 2: Preparation of reference electrodes, preparation of reactors for experiment start, and L. plantarum sub-culturing
- Prepare Ag/AgCl reference electrodes.
NOTE: These steps describe the preparation of the Ag/AgCl reference electrodes indicated in the Table of Materials. Users should follow the manufacturer's instructions if using a different reference electrode.- Disassemble Ag/AgCl reference electrodes and sand the wires. Gently pull the electrode from its glass housing and flick the glass housing to empty the old KCl solution. Gently sand the electrode wires with aluminum oxide sandpaper until evenly shiny to remove oxidized material.
- Collect all sanded reference electrode wires in a small beaker with a small stir bar and fill the beaker with 100% bleach until the wires are fully submerged. Chloride the wires by allowing the wires to bleach on a stirring platform for 30 min until the electrodes turn darker gray. After bleaching, rinse the electrode wires thoroughly with DI water.
- To re-assemble a reference electrode, use a syringe to fill the glass housing fully with 3 M KCl solution saturated with silver chloride, gently tapping the side to dislodge any air bubbles. Using the same syringe, fill the electrode wire cap with KCl solution, then insert the wire into the housing. Place the bottom of the glass housing against a paper towel on the bench top, insert the electrode into the glass housing, then press firmly down on the cap to close the electrode. Store the reference electrodes in a beaker shallowly filled with KCl solution until needed and repeat with all remaining reference electrodes.
- Use a digital multimeter to measure the voltage of homemade Ag/AgCl reference electrodes.
- Submerge the ends of the Ag/AgCl reference electrodes (estimated at 197 mV versus the standard hydrogen electrode, SHE) shallowly in a beaker filled with 3 M KCl. Securely connect the multimeter to a commercially purchased saturated calomel electrode (SCE, 241 mV versus SHE) also submerged in the same KCl electrolytes.
- Measure the potential difference between each reference electrode and the SCE. Confirm that the reference electrodes differ from the SCE by 44 ± 10 mV. Disassemble and reassemble any reference electrode that falls outside this range.
- Seal the seam where the cap meets the glass housing with parafilm.
- Prepare the reactors for experimentation.
- In a sterile biosafety cabinet, exchange the autoclaved water in the reactors with the appropriate media. Pour out the autoclaved water. Fill the cathodic chambers with 110 mL of autoclaved M9 medium. Fill the anodic chambers with 110 mL of freshly prepared mCDM.
- Install the reference electrodes. Remove one GL14 cap from each anodic chamber and replace it with an autoclaved electrode cap (GL14 cap with a silicone sealing ring). Spray the reference electrodes with 70% ethanol to sterilize, then place one reference electrode through the electrode cap into each anodic chamber.
NOTE: Ensure the reference electrode does not make direct contact with the carbon felt round. - Before removing reactors from the biosafety cabinet, tighten all caps and clamps to be finger-tight to avoid leaking.
- Attach the reactors to the water pump system. Place each reactor on the appropriate stir bar platform. Connect the water jacket spigots of each reactor to the next with rubber tubing, connecting end reactors to the inflow and outflow tubes of the water pump.
NOTE: Ensure all connections are tight without leaks, using zip ties to secure tubing if necessary. - Fill the pump with water and add 4-6 drops of water conditioner. Turn the pump system on and set the temperature to 30 ˚C. Start the pump and observe the water flow through all reactor water jackets, ensuring there are no leaks at any connections.
- Turn on the stir platforms and set them to continuous stirring at 220 RPM.
- Attach the reactors to the nitrogen-sparging gas lines. Attach an air filter to a 4 inch, 22 G needle and insert the needle through the top septum of a reactor anodic chamber into the media to serve as the nitrogen inlet. Insert another 1 inch, 18 G needle into the top septum of the anodic chamber to serve as the nitrogen outlet. Connect gas lines from a nitrogen source to the air filter and open the valve to allow gas to bubble gently through the reactor. Ensure that nitrogen bubbles through all anodic chambers continuously throughout the experiment to maintain anaerobic conditions.
NOTE: Ensure the inlet needle is set away from the carbon felt round. The bubble stream should not make contact with either the carbon felt or reference electrodes. - Attach the bioreactors to the potentiostat leads. Connect the working, counter, and reference electrode alligator clip leads from the potentiostat to their corresponding electrodes.
NOTE: Use a multimeter to check the resistance between the wire/current collector and the electrodes to ensure proper electrical connections and minimize potential measurement errors.
- Input potentiostat parameters for prerun.
NOTE: Critical technique settings are given below. See Table 4 for an extended list of software settings for each technique.- Turn on the potentiostat and initialize the EC-lab software on the computer. Connect the potentiostat to the computer by clicking the potentiostat symbol button in the top left panel under Devices. Once connected, the device name will appear with a green circle in the text box below.
- Synchronize all channels connected to the bioreactors into one group by clicking the Edit tab, then selecting synchronize. Click the appropriate channel number boxes | ok.
- Add the technique open circuit voltage (OCV) to the potentiostat by clicking the + button in the Parameters Settings panel on the left. In the blue settings box; set it to measure working electrode potential (EWE) against the reference electrode (RE), recording time in interval dt every 36 s for a total of 3 h.
- Next, add the technique cyclic voltammetry (CV) to the potentiostat. Set the initial potential of the EWE to Ei of 0 V versus RE with a scan rate of 2 mV/s. Sweep to a vertex potential (E1) of 0.4 V versus RE reversing to vertex potential (E2) of -0.7 V versus RE. Repeat the scan for a total of two sweeps.
NOTE: In microbial electrochemical systems, microbial inoculation and biofilm formation induce high capacities compared to metal materials or inorganic molecules. During cyclic voltammetry scanning, the potential varies, and a charging current acts as a background. To achieve a high signal-to-noise ratio, lower scan rates are necessary, though this increases the duration of the scan. Since we scan two cycles covering a wide electrochemical window of 1.1 V, a scan rate of 2 mV/s results in a half cycle taking up to 9.1 min. Consequently, two cycles take a total of 36.4 min. Reducing the scan rate further would be excessively time-consuming. - Add the technique chronoamperometry (CA). Apply a constant potential EWE of 0.2 V versus RE for a time of t = 200 h, recording time in intervals dt every 25-40 s. Adjust the time interval based on potentiostat software to obtain the desired level of detail.
NOTE: The midpoint potential of DHNA is approximately -0.093 V vs. Ag/AgCl; thus, an applied potential of 0.2 V versus RE to the working electrode is sufficient to allow electron transfer from DHNA to the electrode. - After inputting all parameters, begin the run by pressing the green start triangle. Save the file as desired as directed by the software, then click Save. The software will begin technique 1, "OCV." Observe the OCV traces for a few minutes to ensure all reactors read positively and close together with a steady signal. Leave the experiment to run overnight to complete OCV and initial CV (see Supplemental Figure S1 for the media control CV) and allow CA to run until stabilized.
- Under sterile conditions, subculture the MRS culture of L. plantarum 1:200 in 50 mL of mMRS. Grow the cells overnight at 37 ˚C without shaking.
NOTE: A 50 mL overnight culture typically produces more than enough cells for six reactors, assuming a final OD600 of 0.2 in the reactor. Adjust culture volumes accordingly for larger or smaller experiments.
4. Day 3: Injection of cells and DHNA/DMSO
- Wash the cells and inject them into reactors.
- Remove the mMRS L. plantarum culture from the incubator in the morning. Under sterile conditions, transfer the culture to a 50 mL conical tube and place the culture on ice.
- Wash the cells 2x in sterile, cold 1x PBS. To do so, centrifuge the culture at 4,000 × g for 5 min in a 4 ˚C centrifuge to pellet the cells. Under sterile conditions, gently but thoroughly resuspend the cells in 50 mL of PBS, then centrifuge again as before; repeat for a second washing. After the final centrifuge, resuspend the cells in cold PBS to OD600 = 11.
- Under sterile conditions, load 2 mL of resuspended cells into a 3 mL syringe fitted with a needle for each reactor.
- At the reactor station, decap a cell syringe and insert the needle into the top of a reactor anodic chamber. Do not depress the syringe plunger at this time; repeat for all reactors. Once all syringes are in place, depress the plungers to inject the cells into the reactors and record the time of injection from the CA trace. This volume of cells produces a final OD600 of 0.2 in the reactors. Dispose of all syringes in biohazard boxes and needles in the designated sharps biohazard container. Allow the current to stabilize to flat on the CA trace for 2-4 h.
NOTE: Upon injection, current fluctuations can be observed on the CA trace. After 2-4 h, these fluctuations will stabilize to a flat current (<2 µA change in current over the course of an hour), at which point DHNA can be injected.
- Measure initial pH and inject DHNA.
- Prepare a DHNA solution. In a 1.5 mL tube, prepare 500 µL of a 20 mg/mL DHNA solution by dissolving powdered DHNA in 100% DMSO. Fill 3 insulin syringes with 110 µL of DHNA solution and 3 insulin syringes with 110 µL of DMSO only.
NOTE: While DHNA is mildly soluble in water, DMSO is a better solvent for DHNA stocks at this concentration. Mediator solvent may vary if other mediators are used. - At the BES reactor station, label the experimental reactors as + DHNA and the solvent control reactors as - DHNA. Decap a DHNA syringe and insert the syringe into the top of each anodic chamber designated as + DHNA. Insert a DMSO-only syringe into each anodic chamber designated as - DHNA. Do not depress the syringe plungers at this time.
- Take 0 h time point samples for sample analysis. Using a 3 mL syringe fitted with a 2 inch, 21 G needle, take a 2 mL media sample from each anodic chamber through the unused small cap septum and transfer the samples to a 24 deep-well plate to take pH measurements for the 0 h time point (DHNA injection point). If desired, take 1 mL of spent media from each anodic chamber and filter through a 0.2 µm filter into clean labeled tubes for quantifying metabolites using HPLC or other assays. Store spent media samples at -80 ˚C.
- Depress the plungers of all DHNA and DMSO syringes to inject into the reactors. Record the time of injection from the CA trace. Discard all syringes and needles appropriately.
- Measure and record the 0 h pH samples for each reactor.
- Prepare a DHNA solution. In a 1.5 mL tube, prepare 500 µL of a 20 mg/mL DHNA solution by dissolving powdered DHNA in 100% DMSO. Fill 3 insulin syringes with 110 µL of DHNA solution and 3 insulin syringes with 110 µL of DMSO only.
5. Day 4: Experiment completion and sample collection
- Conduct electrochemical analysis 24 h after DHNA injection and take final samples.
- End the CA run 24 h after DHNA injection.
- Take 24 h time point samples for sample analysis according to step 4.2.3.
- Run CV again for the 24 h time point according to the parameters described in step 3.3.4.
- Measure and record the pH for the 24 h samples from each reactor.
- Disassemble and clean the reactors.
- Turn off the potentiostat. Then, disconnect the working, counter, and reference leads from each reactor. Wipe any moisture from the potentiostat lead alligator clips.
- Turn off the nitrogen gas flow. Disconnect the gas leads, and then remove the inflow and outflow needles. Dispose of all needles appropriately in a sharps container.
- Turn off the water pump. Disconnect inflow and outflow tubing from the pump and allow water to drain into a bucket. When disconnecting, keep the tubing ends elevated above the water line in the pump to prevent water from siphoning onto the floor. One by one, disconnect each reactor from the water jacket tubing, working from the final outflow reactor to the initial inflow reactor.
- Empty all media from the reactors into a large biohazard container. Follow normal culture bleaching methods for disposal.
- Once emptied, disassemble and clean all reactor pieces. Dispose of cation exchange membranes and carbon felt rounds into proper biohazard waste. Clean reference electrodes and titanium wires with 70% ethanol and store the cleaned reference electrodes in a beaker shallowly filled with water. Gently clean reactor bottles, caps, O-rings, and clamps in warm water with laboratory detergent, rinse thoroughly with DI water, and air dry all parts before storing.
6. Day 5: Electrochemical analysis
NOTE: Below is a general description of data plotting for this protocol. More detailed descriptions concerning analysis and data interpretation will be provided in the Representative Results section.
- For CA analysis: Set the time of DHNA injection as the 0 h time point. Plot the average and standard deviation of the measured current density (j in µA/cm2) taken every 36 s for all replicates from the 0 h time point as a function of time (h). Calculate current density as a function of the working electrode area (16 cm2).
- For CV analysis: Plot a representative CV trace for each experimental condition (media only, DMSO, or DHNA) depicting current density (j in µA/cm2) as a function of the working electrode potential (EWE in V). Plot cycle two of the selected CV run.
Representative Results
Chronoamperometry analysis
The EET of L. plantarum can be observed through the chronoamperometry (CA) data depicted in Figure 3, in which the current density trace visualizes electron transfer from L. plantarum to the working electrode. We monitored the current density (j) versus time while maintaining a constant potential of +200 mV versus Ag/AgCl for 24 h. Upon injecting 20 µg/mL DHNA into the stirring electrolyte solution, an abiotic DHNA oxidation spike was observed, followed by a rapid increase in biotic current density peaking at 132.0 ± 2.47 µA/cm2 at approximately the 8 h time point. Conversely, the injection of DMSO resulted in negligible current density. These results emphasize the significance of DHNA as a necessary and efficient mediator to facilitate electron transfer between L. plantarum and the electrode. Users can adjust the current output by adjusting the concentration of DHNA in the BES. Prior research also indicates L. plantarum responds to DHNA in a dose-dependent manner across a wide range of DHNA concentrations, producing significant current in the presence of DHNA concentrations as low as 0.01 µg/mL13,22.
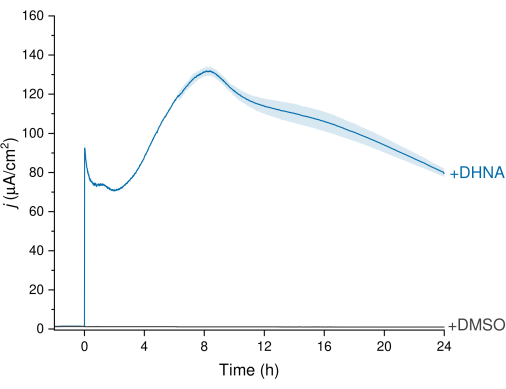
Figure 3: Chronoamperometry analysis of Lactiplantibacillus plantarum EET mediated by DHNA. DHNA (20 µg/mL) or DMSO was injected into mCDM electrolytes (pH~ 6.5) with injection time identified as t = 0. j represents current density as a function of the working electrode area. Experiments were conducted at 200 mV versus Ag/AgCl with a carbon felt electrode (16 cm2) and stirring. Values are plotted as mean ± sd obtained in triplicate BES reactors. Abbreviations: EET = extracellular electron transfer; DHNA = 1,4-dihydroxy-2-naphthoic acid; DMSO = dimethyl sulfoxide; mCDM = Chemically Defined Medium with mannitol. Please click here to view a larger version of this figure.
Cyclic voltammetry analysis
To further evaluate DHNA-mediated EET in L. plantarum, we conducted cyclic voltammetry 24 h after DHNA injection. Here we show CV traces for three conditions: L. plantarum with 20 µg/mL DHNA, L. plantarum with DMSO, and media with 20 µg/mL DHNA. As shown in Figure 4A, the presence of 20 µg/mL DHNA in reactors containing L. plantarum resulted in a distinct increase in oxidative current at 50 mV that did not occur in the presence of DMSO alone. These data confirm that adding the redox mediator DHNA is necessary to facilitate electron transfer between L. plantarum and the anode. While we observed various smaller redox peaks in the L. plantarum + DMSO trace, these peaks were similar to the media control trace and are likely attributed to redox-active components in mCDM (Supplemental Figure S1). In Figure 4B, we compared traces of DHNA under biotic conditions (L. plantarum + DHNA) versus DHNA under abiotic conditions (Media + DHNA). While both traces exhibited a distinct DHNA oxidative peak around 50 mV, we observed a sustained increase in current beyond 50 mV only under biotic conditions. The catalytic peak reached a current density of 129 µA/cm2 at 300 mV, representing a 256% increase compared to the abiotic trace. This turnover CV profile is characteristic of microbial EET27, indicating re-reduction of DHNA by L. plantarum cells in the presence of an electron source (mannitol) after oxidation of DHNA at the anode. Additionally, the abiotic trace exhibited new oxidative peaks around -240 mV and -180 mV. Prior research indicates the appearance of these peaks may be due to the degradation of DHNA into ACNQ (2-amino-3-carboxy-1,4-naphthoquinone)21,28. We did not observe these peaks in the biotic trace, indicating that the interaction of L. plantarum cells with DHNA may stabilize DHNA and prevent degradation. A point to note is that the 24 h trace for media with 20 µg/mL DHNA was conducted separately according to this protocol without adding cells.
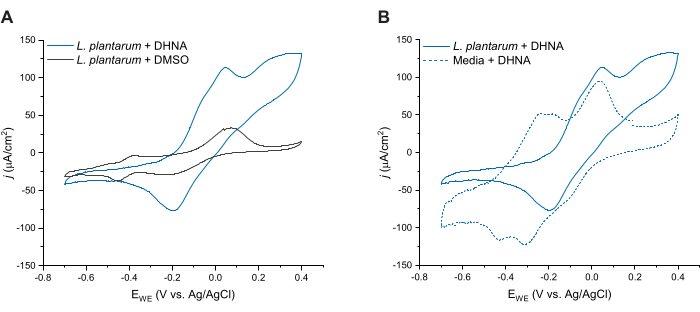
Figure 4: Representative cyclic voltammetry traces. All CV experiments were performed in mCDM using carbon felt (16 cm2) as the working electrode at a scan rate of 2 mV/s while stirring the solution. (A) CV traces for Lactiplantibacillus plantarum with either DHNA (20 µg/mL) or DMSO at t = 24 h. (B) CV traces of 20 µg/mL DHNA in either L. plantarum (biotic conditions) or mCDM only (abiotic conditions) at t = 24 h. Abbreviations: CV = cyclic voltammetry; mCDM = Chemically Defined Medium with mannitol; DHNA = 1,4-dihydroxy-2-naphthoic acid; DMSO = dimethyl sulfoxide. Please click here to view a larger version of this figure.
pH analysis
EET activity in L. plantarum resulted in a notable drop in pH over 24 h. As shown in Figure 5, the average sample pH of L. plantarum exposed to DHNA dropped to 3.33 ± 0.01 (p = 6.85 × 10-6, n = 3), while the average sample pH of L. plantarum exposed to DMSO dropped to 6.50 ± 0.06 (p = 0.0409, n = 3). As exhibited in prior research, this drop is attributed to an increase in fermentative metabolism that occurs when L. plantarum performs EET13. L. plantarum normally metabolizes mannitol through glycolysis and fermentative pathways, which produce acetate, lactate, and ethanol as end-fermentation products and generate ATP through substrate-level phosphorylation29. Under EET conditions, metabolic flux through fermentation increases, thereby increasing the production of end-fermentation products in the BES media13. This metabolic shift causes the media pH to drop more rapidly in the reactors with DHNA compared to the DMSO control reactors.
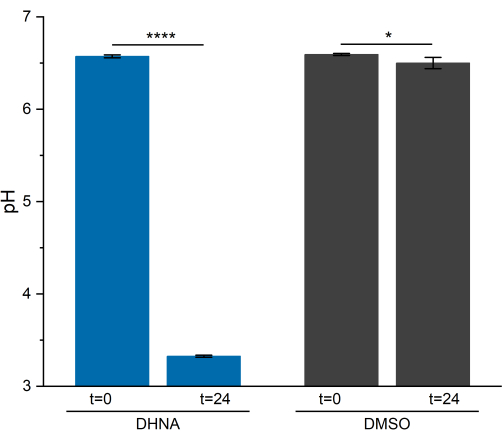
Figure 5: pH analysis of Lactiplantibacillus plantarum bioelectrochemical system. Samples were collected at t = 0 and t = 24 h during chronoamperometry. Values are plotted as mean ± sd obtained in triplicate BES reactors. Significance was determined by a one-tailed t-test. DHNA: P-value = 6.85 × 10-6. DMSO: P-value = 0.0409. Abbreviations: DHNA = 1,4-dihydroxy-2-naphthoic acid; DMSO = dimethyl sulfoxide. Please click here to view a larger version of this figure.
Table 1: Ingredients for preparation of mMRS media24. Please click here to download this Table.
Table 2: Ingredients for preparation of mCDM media. This table is taken from Tejedor-Sanz et al.13 and Aumiller et al.25. Please click here to download this Table.
Table 3: Ingredients for preparation of M9 media. Please click here to download this Table.
Table 4: EC-Lab parameter settings for OCV, CA, and CV techniques. Abbreviations: OCV = open circuit voltage; CA = chronoamperometry; CV = cyclic voltammetry. Please click here to download this Table.
Supplemental Figure S1: Representative cyclic voltammetry traces of Lactiplantibacillus plantarum with DMSO and mCDM alone. CV traces for L. plantarum with DMSO at t = 24 h and mCDM alone at t = 0 h. All CV experiments were performed using carbon felt (16 cm2) as the working electrode at a scan rate of 2 mV/s while stirring the solution. Abbreviations: CV = cyclic voltammetry; mCDM = Chemically Defined Medium with mannitol; DHNA = 1,4-dihydroxy-2-naphthoic acid; DMSO = dimethyl sulfoxide. Please click here to download this File.
Discussion
Using the three-electrode, two-chamber bioelectrochemical system described here, we showed measurement of current generation from DHNA-mediated EET in L. plantarum. These BES experiments generate high-quality data; however, BESs are sensitive. Thus, protocol success depends on user precision, particularly in reactor and reference electrode assembly, positioning of needles and electrodes within the anodic chamber, and cation exchange membrane replacement. It is critical to assemble reactors carefully, ensuring no water/media leakage during autoclaving or experimentation. Water leakage can be resolved by ensuring cation exchange membranes are cut to precisely fit the O-ring and tightening the knuckle clamp to finger tight. It is also essential to fully submerge the carbon felt round fully in water during autoclaving to allow it to become hydrophilic for experimentation. We recommend new users allow newly assembled reactors filled with water to sit for 2 h prior to autoclaving, checking for signs of slow leaks below the main bottle junctions. Moreover, ensuring a proper reference electrode assembly guarantees consistent data replication across reactors. If the Teflon frit inside the glass housing becomes discolored, cracked, or dried, this can cause a high resistance of the reference electrode. Users can replace the glass housing to restore reference electrode performance.
Proper orientation of all needles and electrodes within the anodic chamber during experimentation is critical to experiment success. The reference electrode must not directly contact any part of the carbon-felt working electrode. Users can adjust the carbon felt position by gently rotating the working electrode titanium wire from above the reactor. Additionally, needle placement for nitrogen sparging should not make direct contact with electrodes within the chamber or any electrode/potentiostat connections above the chamber. The nitrogen stream should be adjusted to not flow into either electrode. Finally, users should make sure the stir bar does not contact the working electrode by positioning the working electrode 1-2 cm above the stir bar. If an erratic signal is observed in OCV, this can usually be resolved by ensuring proper placement of electrodes and the nitrogen stream within the reactor, and by checking that connections between the potentiostat leads and the reactor electrodes are correct and secure. Lastly, our experience shows that electron mediators like DHNA can be retained within the cation exchange membrane and cause a high background current if reused too many times. We recommend replacing the cation exchange membrane after two to three uses, especially when investigating mediated EET, to guarantee reliable experimental results.
Unlike direct EET, where direct microbial attachment to the electrode facilitates electron transfer, mediated EET necessitates consistent diffusion of electron shuttles across the cell membrane and the electrode, resulting in the unique BES settings described here. First, we chose a double-chamber BES over the single-chamber counterpart in our protocol to separate anodic and cathodic reactions with a cation exchange membrane. This separation prevents the freely diffusing electron mediators (DHNA) and microbes from cross-interacting with the cathode, ensuring microbial EET is the major electron source to reduce the electron mediators and the anode. The separation also allows precise control over parameters like mediator concentration/distribution and the potential poised to the anode. Additionally, we chose carbon felt as the anode material amongst other options such as graphite rods, metal electrodes, glassy carbon, or indium tin oxide (ITO). This is because the 3D porous structure of carbon felt provides a much larger surface area than those electrodes30, allowing efficient utilization of mediators even at high concentrations. Our three-electrode, two-chamber BES settings provide a reliable and reproducible readout of mediated EET even over long-term monitoring; however, this process is relatively low throughput. This protocol is suitable for bench-scale understanding of EET mechanisms or to test prototype EET applications. Alternative BES architectures such as portable or printed BESs31,32, complementary metal oxide semiconductor (CMOS) arrays33, or up-scaled BESs34 can be considered by researchers for different fundamental or application purposes.
In this protocol, we provide detailed instructions for the most commonly used electrochemical techniques: chronoamperometry (CA) and cyclic voltammetry (CV). It is worth noting that other electrochemical techniques, such as Electrochemical Impedance Spectroscopy (EIS) and Differential Pulse Voltammetry (DPV), can provide deeper insights into the BES by analyzing charge transfer resistance and double-layer capacitance35,36,37. While this BES protocol enables EET measurements, complementing electrochemical data with metabolic activity and cell biomass measurements can also be essential for a comprehensive analysis. Microbes like L. plantarum engage EET as one of the electron sinks alongside other fermentation byproducts such as lactate and ethanol. Moreover, it is noteworthy that cell biomass growth also serves as an electron sink13. Therefore, quantifying consumed electron donors (e.g., mannitol), assessing cell biomass growth, and monitoring fermentation byproducts offer deeper insights into the efficiency and physiological ramifications of EET. Cellular metabolites are commonly quantified using chromatography and enzymatic assays, while cell viability and growth are assessed by counting colony-forming units and measuring the optical density of spent media at 600 nm, respectively13. It is also important to note that EET measurements are sensitive to small perturbations in experimental conditions. This includes but is not limited to pH, temperature, stirring speed, and nitrogen gas sparging rate38. Therefore, normalizing measured EET levels with bioanalytical measurements acts as an internal control, facilitating consistent assessment across experiments conducted on different days.
Combining electrochemical techniques with other bioanalytical measurements, mediated EET creates new opportunities for electro-fermentation and bioelectrocatalysis. Conventional use of organic, inorganic, or enzymatic electrocatalysts poses challenges due to their high cost and are prone to degradation. Alternatively, using microbes as living electrocatalysts offers a less expensive and more scalable solution due to microbes' self-repair and self-replication abilities39. L. plantarum, generally recognized as a safe lactic acid bacterium, is a particularly intriguing chassis. Using identical electrochemical setups described in this protocol, we have previously shown that L. plantarum can ferment kale juice under EET conditions and accelerate the metabolic flux toward producing more fermentation end-products such as lactate, acetate, and succinate13; these organic acids are essential flavor compounds in food fermentation. This implies that, by using electrochemical techniques, mediated EET in L. plantarum can be potentially hijacked to manipulate metabolic flux, alter food flavors, or produce valuable chemicals. It is worth noting that the electrochemical techniques presented in this protocol can not only be applied to L. plantarum but can also be generically applied to other native or engineered microbes that perform mediated EET40,41. Different electron mediators, such as flavin, ferrocene, neutral red, ferricyanide, lawsone, and menadione can be selected based on the electron transfer mechanism of the specific microbe being used22,42. Moreover, the BES protocol established in this work can be extended to exoelectrogens that perform mediatorless EET as previously demonstrated with Shewanella and Geobacter species43,44. An optimized growth medium should be used to support the cellular activity of the particular microbe to facilitate its EET performance. This protocol fine-tunes parameters for DHNA-mediated EET in L. plantarum, but modifications are expected when a different microbe and electron mediators are applied.
Acknowledgements
We thank members of the Ajo-Franklin lab for insightful discussions on BES assembly, maintenance, critical steps, and troubleshooting. The research was sponsored by the Army Research Office and was accomplished under Grant Number W911NF-22-1-0239 (to C. M. A-F, supporting R. A.) and by the Cancer Prevention and Research Institute of Texas, grant # RR190063 (to C. M. A-F, supporting R. C., S. L., and B. B. K.). Figure 1 was created with BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 1,4-Dihydroxy-2-naphthoic acid (DHNA) | Sigma-Aldrich | 281255-25G | |
| 1.0 mm diameter titanium wire | Thermo Fisher Scientific | 045485.BY | Cut to size for working and counter electrodes |
| 120-C Aluminum Oxide Sheets 9" x 11" | Johnson Abrasives | 10108-15 | |
| 3 mL plastic syringes | Thermo Fisher Scientific | 14955457 | |
| 3M KCl solution saturated with silver chloride | Millipore Sigma | 60137-250ML | |
| 6.35-mm-thick carbon felt | Thermo Fisher Scientific | 043200.RF | Cut into 16 cm2 rounds |
| Ag/AgCl reference electrode | CH Instruments | CH111 | |
| Air-Tite Premium Hypodermic Needles | Thermo Fisher Scientific | 14-817-102 | |
| AlK(SO)4 * 12H2O | Sigma-Aldrich | 237086-100G | |
| Ammonium citrate tribasic | Millipore Sigma | A1332 | |
| Avanti J-15R Centrifuge | Beckman Coulter | B99517 | |
| BD Precision Glide Needle, 18 G x 1 inch | Thermo Fisher Scientific | 14-826-5G | |
| BD Precision Glide Needle, 21 G x 2 inch | Thermo Fisher Scientific | 14-821-13N | |
| Bel-Art SP Scienceware Cleanware Aqua-Clear Water Condtioner | Thermo Fisher Scientific | 23-278339 | |
| Biotin | Millipore Sigma | B4639 | |
| CaCl2 | Millipore Sigma | C4901 | |
| Calcium D-(+)-pantothenate | Millipore Sigma | 1087009 | |
| Casamino acids | Millipore Sigma | 2240-OP | |
| cation exchange membrane | Membranes International | CMI-7000 | Cut into rounds fit to the BES O-ring |
| CoCl2 * 6H2O | Millipore Sigma | C8661 | |
| CuSO4 * 5H2O | Millipore Sigma | C8027 | |
| Cysteine-HCl * H2O | Millipore Sigma | 30129 | |
| DMSO | Millipore Sigma | 5439001000 | |
| DS-11+ Spectrophotometer | Denovix | N/A | |
| EC-Lab Software | BioLogic | N/A | |
| ECO E 4 S heating circulator | Lauda-Brinkmann | Cat. No. 115 V; 60 Hz : L001191 | |
| FeSO4 * 7H2O | Millipore Sigma | 215422 | |
| Folic acid | Millipore Sigma | F8758 | |
| H3BO3 | Millipore Sigma | B6768 | |
| Insulin syringes with BD Micro-Fine IV Needle | Thermo Fisher Scientific | 14-829-1A | |
| Lactiplantibacillus plantarum NCIMB8826 | N/A | N/A | Reference: Tejedor-Sanz et al., 2022 |
| Lactobacillus MRS Broth | HiMedia | M369 | |
| M9 Broth | Milliport Sigma | 63011 | |
| Magnesium sulfate anhydrous | Millipore Sigma | 208094 | |
| Manganese sulfate monohydrate | Millipore Sigma | 221287 | |
| mannitol | Millipore Sigma | M1902-1KG | |
| Mettler Toledo FiveEasy Benchtop pH Meter | Hogentogler | F20-KIT | |
| MgCl2 * 6H2O | Millipore Sigma | M9272 | |
| MgSO4 * 7H2O | Millipore Sigma | M2773 | |
| Millex - GV 0.22 µm PVDF Membrane Filter Unit | Millipore Sigma | SLGV004SL | |
| MnCl2 * 4H2O | Millipore Sigma | 203734 | |
| MnSO4 * H2O | Millipore Sigma | 221287 | |
| MOPS | Millipore Sigma | M1442 | |
| N2 gas | Airgas | NI UHP300 | Filter before use |
| Na2MoO4 * 2H2O | Millipore Sigma | 331058 | |
| Na2SO4 | Millipore Sigma | 238597 | |
| NaCl | Millipore Sigma | S9888 | |
| NH4Cl | Millipore Sigma | A9434 | |
| Nicotinic acid | Millipore Sigma | N-0761 | |
| Nitrilotriacetic acid (NTA) | Millipore Sigma | 72560 | |
| p-Aminobenzoic acid | Millipore Sigma | P9879 | |
| Phosphate buffered saline, 10x solution | Thermo Fisher Scientific | BP399-1 | |
| Potassium phosphate dibasic | Millipore Sigma | P8281 | |
| potentiostat | BioLogic | VMP-300 | |
| Protease peptone #3 | Bacto | 211693 | |
| Pyridoxine HCl | Millipore Sigma | P6280 | |
| Riboflavin | Millipore Sigma | 555682 | |
| RO10 magnetic stir bar platform | IKA | 3691000 | |
| Sodium acetate trihydrate | Millipore Sigma | 935700 | |
| Stir bar, egg-shaped | Thermo Fisher Scientific | 14-512-121 | Place in anodic chamber of BES |
| Thiamine HCl | Millipore Sigma | V-014 | |
| Thioctic acid (α-Lipoic acid) | Millipore Sigma | T-1395 | |
| Tryptophan | Millipore Sigma | 9136 | |
| Tween80 | Millipore Sigma | P4780 | |
| Vitamin B12 | Millipore Sigma | V6629 | |
| Jacketed MCF set, 100 ml, NW25, 2 x GL14 port | Adams & Chittenden Scientific Glass | NA | Customized |
| Yeast extract | Millipore Sigma | Y1625 | |
| ZnSO4 * 7H2O | Millipore Sigma | Z0251 |
References
- TerAvest, M. A., Ajo-Franklin, C. M. Transforming exoelectrogens for biotechnology using synthetic biology. Biotechnol Bioeng. 113 (4), 687-697 (2016).
- Chen, H., Dong, F., Minteer, S. D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials. Nat Catal. 3 (3), 225-244 (2020).
- Chen, H., et al. Fundamentals, applications, and future directions of bioelectrocatalysis. Chem Rev. 120 (23), 12903-12993 (2020).
- Lovley, D. R., Phillips, E. J. P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 54 (6), 1472-1480 (1988).
- Myers, C. R., Nealson, K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 240 (4857), 1319-1321 (1988).
- Koch, C., Harnisch, F. Is there a specific ecological niche for electroactive Microorganisms. ChemElectroChem. 3 (9), 1282-1295 (2016).
- Zhao, J., et al. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol Adv. 53, 107682 (2021).
- Zhang, J., Li, F., Liu, D., Liu, Q., Song, H. Engineering extracellular electron transfer pathways of electroactive microorganisms by synthetic biology for energy and chemicals production. Chem Soc Rev. 53 (3), 1375-1446 (2024).
- Logan, B. E. Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol. 7 (5), 375-381 (2009).
- Logan, B. E., Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science. 337 (6095), 686-690 (2012).
- Gu, L., Xiao, X., Yup Lee, S., Lai, B., Solem, C. Superior anodic electro-fermentation by enhancing capacity for extracellular electron transfer. Bioresour Technol. 389, 129813 (2023).
- Boucher, D. G., et al. Bioelectrocatalytic synthesis: concepts and applications. Angew Chem Int Ed. 62 (46), e202307780 (2023).
- Tejedor-Sanz, S., et al. Extracellular electron transfer increases fermentation in lactic acid bacteria via a hybrid metabolism. eLife. 11, e70684 (2022).
- Martino, M. E., et al. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environ Microbiol. 18 (12), 4974-4989 (2016).
- Fidanza, M., Panigrahi, P., Kollmann, T. R. Lactiplantibacillus plantarum-nomad and ideal probiotic. Front Microbiol. 12, 712236 (2021).
- Duar, R. M., et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev. 41 (Suppl_1), S27-S48 (2017).
- Kaushik, J. K., et al. Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLOS ONE. 4 (12), e8099 (2009).
- Seddik, H. A., et al. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob Proteins. 9 (2), 111-122 (2017).
- Siezen, R. J., et al. Phenotypic and genomic diversity of Lactobacillus plantarum strains isolated from various environmental niches. Environ Microbiol. 12 (3), 758-773 (2010).
- Light, S. H., et al. A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature. 562 (7725), 140-144 (2018).
- Tolar, J. G., Li, S., Ajo-Franklin, C. M. The differing roles of flavins and quinones in extracellular electron transfer in Lactiplantibacillus plantarum. Appl Environ Microbiol. 89 (1), e0131322 (2023).
- Li, S., De Groote Tavares, C., Tolar, J. G., Ajo-Franklin, C. M. Selective bioelectronic sensing of pharmacologically relevant quinones using extracellular electron transfer in Lactiplantibacillus plantarum. Biosens Bioelectron. 243, 115762 (2024).
- Blackburn, B., Alba, R., Hatch, A., Ajo-Franklin, C. M., Mevers, E. Understanding the Chemical Properties that Drive Extracellular Electron Shuttles. ChemRxiv. , (2024).
- De Man, J. C., Rogosa, M., Sharpe, M. E. A medium for the cultivation of Lactobacilli. J Appl Bacteriol. 23 (1), 130-135 (1960).
- Aumiller, K., et al. A chemically-defined growth medium to support Lactobacillus-Acetobacter sp. community analysis. PLOS ONE. 18 (10), e0292585 (2023).
- Brooijmans, R. J. W., de Vos, W. M., Hugenholtz, J. Lactobacillus plantarum WCFS1 electron transport chains. Appl Environ Microbiol. 75 (11), 3580-3585 (2009).
- LaBelle, E., Bond, D. R. Cyclic voltammetry for the study of microbial electron transfer at electrodes. Bio-electrochemical systems: from extracellular electron transfer to biotechnological application. , (2009).
- Mevers, E., et al. An elusive electron shuttle from a facultative anaerobe. eLife. 8, e48054 (2019).
- Dirar, H., Collins, E. B. End-products, Fermentation balances and molar growth yields of homofermentative Lactobacilli. Microbiology. 73 (2), 233-238 (1972).
- Huong Le, T. X., Bechelany, M., Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon. 122, 564-591 (2017).
- Zhou, A. Y., Baruch, M., Ajo-Franklin, C. M., Maharbiz, M. M. A portable bioelectronic sensing system (BESSY) for environmental deployment incorporating differential microbial sensing in miniaturized reactors. PLOS ONE. 12 (9), e0184994 (2017).
- Benjamin, S. R., de Lima, F., Nascimento, V. A. d. o., de Andrade, G. M., Oriá, R. B. Advancement in paper-based electrochemical biosensing and emerging diagnostic methods. Biosensors. 13 (7), 689 (2023).
- Kumashi, S. R., et al. A CMOS multi-modal electrochemical and impedance cellular sensing array for massively paralleled exoelectrogen screening. IEEE Trans Biomed Circuits Syst. 15 (2), 221-234 (2021).
- Jadhav, D. A., et al. Scale-up of the bioelectrochemical system: Strategic perspectives and normalization of performance indices. Bioresour Technol. 363, 127935 (2022).
- Kim, J., Cestellos-Blanco, S., Shen, Y., Cai, R., Yang, P. Enhancing biohybrid CO2 to multicarbon reduction via adapted whole-cell catalysts. Nano Lett. 22 (13), 5503-5509 (2022).
- He, Z., Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ Sci. 2 (2), 215-219 (2009).
- Okamoto, A., Hashimoto, K., Nealson, K. H., Nakamura, R. Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones. Proc Natl Acad Sci U S A. 110 (19), 7856-7861 (2013).
- Zhang, X., Li, X., Zhao, X., Li, Y. Factors affecting the efficiency of a bioelectrochemical system: a review. RSC Adv. 9 (34), 19748-19761 (2019).
- Kornienko, N., Zhang, J. Z., Sakimoto, K. K., Yang, P., Reisner, E. Interfacing nature's catalytic machinery with synthetic materials for semi-artificial photosynthesis. Nat Nanotechnol. 13 (10), 890-899 (2018).
- Glasser, N. R., Saunders, S. H., Newman, D. K. The colorful world of extracellular electron shuttles. Annu Rev Microbiol. 71 (1), 731-751 (2017).
- Gemünde, A., Lai, B., Pause, L., Krömer, J., Holtmann, D. Redox mediators in microbial electrochemical systems. ChemElectroChem. 9 (13), e202200216 (2022).
- Kundu, B. B., et al. Extracellular respiration is a latent energy metabolism in Escherichia coli. bioRxiv. , (2024).
- O'Brien, J. P., Malvankar, N. S. A simple and low-cost procedure for growing Geobacter sulfurreducens cell cultures and biofilms in bioelectrochemical systems. Curr Protoc Microbiol. 43 (1), A.4K.1-A.4K.27 (2016).
- Su, L., Fukushima, T., Ajo-Franklin, C. M. A hybrid cyt c maturation system enhances the bioelectrical performance of engineered Escherichia coli by improving the rate-limiting step. Biosens Bioelectron. 165, 112312 (2020).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved