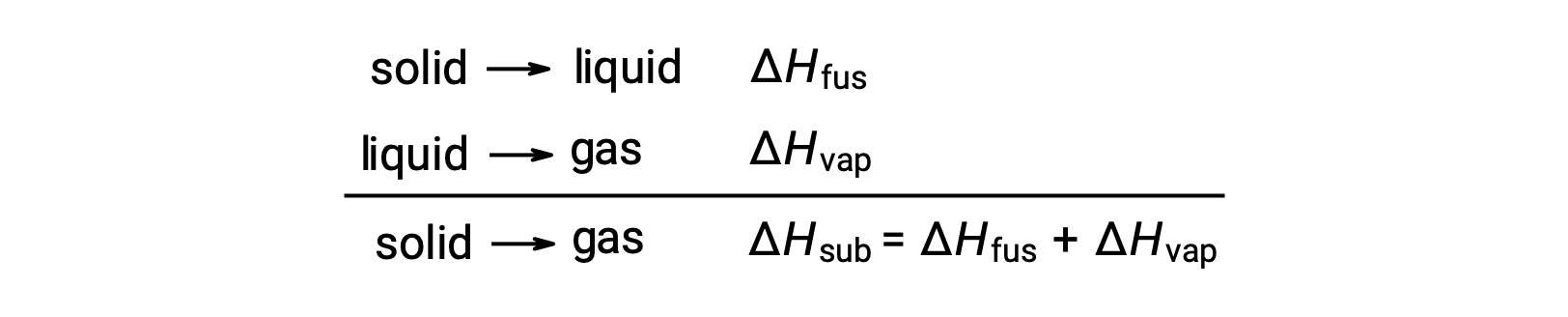It is commonly observed that regular ice melts under ambient conditions, but dry ice does not; instead, dry ice transitions directly into the gas phase. This transition from solid to gas — without passing through the liquid phase — is known as sublimation.
Generally, compounds that sublimate exhibit weak intermolecular forces in the solid state. In dry ice, or solid carbon dioxide, weak dispersion forces exist between CO2 molecules.
At atmospheric pressure, dry ice remains a solid below negative 78.5 °C. However, at −78.5 °C, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and transform directly into the vapor phase. This is the sublimation point of dry ice.
The amount of energy required to sublimate one mole of a solid is called its molar heat of sublimation or its molar enthalpy of sublimation. As sublimation is an endothermic process, its enthalpy value is always positive.
The reverse of sublimation — that is, the direct transition from vapor to solid — is called deposition. When gas molecules collide with cooler solid surfaces, they lose heat. Multiple collisions result in a significant loss of heat, and the molecules ultimately deposit.
Since deposition involves loss of energy, it is an exothermic phase change with a negative enthalpy value. Although the enthalpy of deposition is negative, its magnitude is the same as the enthalpy of sublimation.
When sublimation occurs in an open system, most sublimed molecules disperse in air and never come back. Consequently, the rate of sublimation is greater than the rate of deposition.
However, in a closed system, a solid–vapor equilibrium is established at the solid’s sublimation point.
The partial pressure exerted by the gas in dynamic equilibrium with its solid is called its vapor pressure. Solids that sublimate have high vapor pressures. Dry ice, for example, has a vapor pressure as high as 56.5 atm at 20 °C.
However, since most solids have low vapor pressures at easily accessible temperatures, sublimation is not common.