12.8 : 蒸気圧の低下
液体の平衡蒸気圧とは、気化と凝縮が同じ割合で起こっているときに、その気体相が及ぼす圧力のことです。
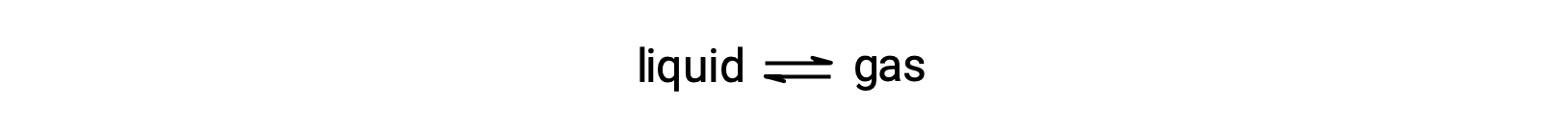
揮発性の液体に不揮発性の物質を溶かすと、液体の蒸気圧は降下します。この現象は、液体の気化・凝縮過程における溶質分子の影響を考慮することで説明できます。気化するためには、溶液の表面に溶媒分子が存在しなければなりません。溶質が存在すると、溶媒分子が利用できる表面積が減少するため、溶媒の気化速度が低下します。凝縮の速度は溶質の存在に影響されないので、正味の結果として、気相中の溶媒分子の数が少ない(つまり、蒸気圧が低い)状態で、気化-凝縮の平衡が達成されることになります。
この解釈は有用ですが、蒸気圧低下の衝突的性質のいくつかの重要な側面を説明していません。より厳密に説明するには、エントロピーという性質を用います。液体の蒸気圧の低下を理解するためには、溶媒と溶質が別々の相であるのに比べて、溶液中の物質はより分散した性質を持っているため、溶媒分子を効果的に安定させ、その気化を妨げる役割を果たしていることに注意すれば十分です。その結果、蒸気圧が低くなり、沸点が高くなります。
溶液成分の蒸気圧とその濃度の関係は、ラウールの法則で表されます。理想的な溶液中の任意の成分が及ぼす分圧は、純粋な成分の蒸気圧に溶液中のモル分率を乗じたものに等しいです。
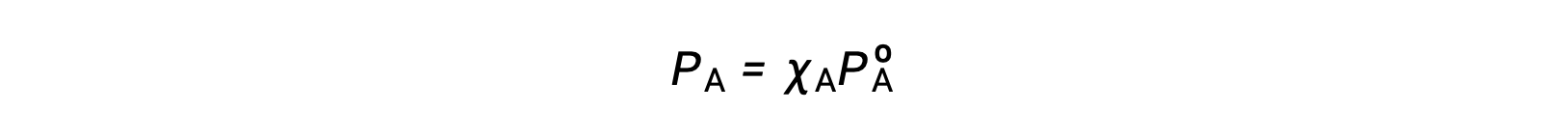
ここで、PAは溶液中の成分Aが及ぼす分圧、PºAは純粋なAの蒸気圧、XAは溶液中のAのモル分率です。
気体混合物の全圧力は、すべての成分の分圧の合計に等しい(ダルトン分圧の法則)とすると、i成分を含む溶液が発揮する全蒸気圧は以下のようになります。
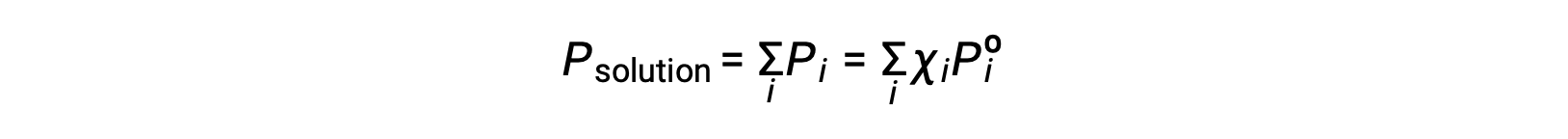
不揮発性物質とは、蒸気圧が無視できるほど小さい(Pº ≈ 0)物質のことで、不揮発性溶質のみを含む溶液の上の蒸気圧は、溶媒のみによるものです。
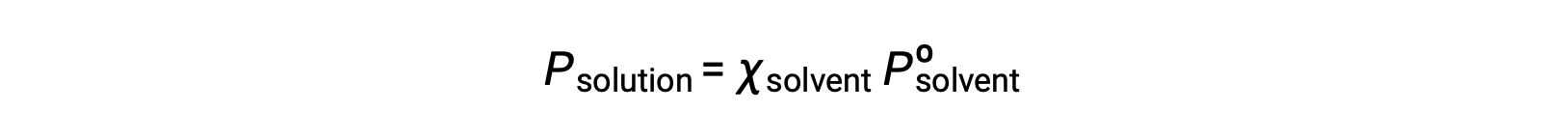
上記の文章は以下から引用しました。Openstax, Chemistry 2e, Section 11.4: colligative properties.
章から 12:

Now Playing
12.8 : 蒸気圧の低下
溶液とコロイド
25.7K 閲覧数

12.1 : 溶液の形成
溶液とコロイド
30.9K 閲覧数

12.2 : 溶液中の分子間力
溶液とコロイド
32.7K 閲覧数

12.3 : 溶液のエンタルピー
溶液とコロイド
24.5K 閲覧数

12.4 : 水溶液及び水和熱
溶液とコロイド
14.2K 閲覧数

12.5 : 溶液の平衡と飽和
溶液とコロイド
18.2K 閲覧数

12.6 : 溶解性に影響する物理特性
溶液とコロイド
22.2K 閲覧数

12.7 : 溶液濃度の表現
溶液とコロイド
58.0K 閲覧数

12.9 : 理想溶液
溶液とコロイド
18.9K 閲覧数

12.10 : 凝固点降下と沸点上昇
溶液とコロイド
33.8K 閲覧数

12.11 : 浸透圧及び溶液の浸透圧
溶液とコロイド
38.7K 閲覧数

12.12 : 電解質:ファントホフ係数
溶液とコロイド
32.5K 閲覧数

12.13 : コロイド
溶液とコロイド
17.2K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved