15.9 : 酸の混合物
酸を含む溶液のpHは、その酸の解離定数と初期濃度を用いて決定することができます。ある溶液に2種類の酸が含まれている場合は、酸の相対的な強さと解離定数に応じて、いくつかの方法でpHを決定することができます。
強酸と弱酸の混合物
強酸と弱酸の混合溶液では、強酸は完全に解離し、溶液中に存在するほとんどすべてのヒドロニウムイオンの供給源となります。一方、弱酸は部分的に解離し、ごくわずかな濃度のヒドロニウムイオンしか生成しません。強酸が生成する高濃度のヒドロニウムイオンは、弱酸の解離をさらに低下させます。これは、ル・シャトリエの原理により、平衡状態にある化学系が乱されると、その乱れを最小にする方向に系が移動するために起こる現象です。その結果、弱酸の解離量が減少します。このため、強酸と弱酸の混合物のpHは、強酸の濃度のみから算出することができます。例えば、強酸である塩酸と弱酸であるギ酸を同じ濃度で混合した場合のpHは、塩酸の濃度のみから求めることができます。混合物中のHClの濃度が0.0020 Mであれば、そのpHは次のように計算できます。
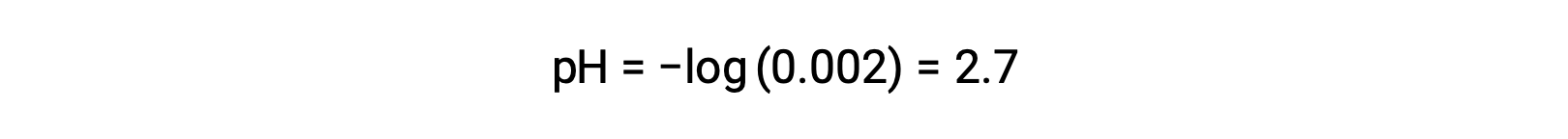
ここでは、HCHO2や水の自己イオン化によって生成されるヒドロニウムイオンの濃度は無視できます。
解離定数の異なる2つの弱酸の混合物
2つの弱酸の混合物において、強酸の解離定数が弱酸よりも著しく大きい場合、混合物のpHは強酸によって決定されます。例えば、同濃度の亜硝酸(HNO2)と次亜塩素酸(HClO)の混合物では、HNO2が主にpHを決定します。亜硝酸のKa(4.6 × 10−4)は次亜塩素酸のKa (2.9 × 10−8)の約1万倍であり、ル・シャトリエの原理によれば、HClOはHNO2の存在下で解離量が減少します。
章から 15:

Now Playing
15.9 : 酸の混合物
酸・塩基
19.5K 閲覧数

15.1 : ブレンステッド・ローリーの酸塩基理論
酸・塩基
90.8K 閲覧数

15.2 : 酸/塩基強度及び解離定数
酸・塩基
60.1K 閲覧数

15.3 : 水:ブレンステッド・ローリーの酸塩基理論
酸・塩基
49.9K 閲覧数

15.4 : pHスケール
酸・塩基
68.3K 閲覧数

15.5 : 共役酸塩基対の相対強度
酸・塩基
45.3K 閲覧数

15.6 : 強酸、強塩基性溶液
酸・塩基
31.4K 閲覧数

15.7 : 弱酸性溶液
酸・塩基
37.6K 閲覧数

15.8 : 弱塩基性溶液
酸・塩基
22.5K 閲覧数

15.10 : 酸・塩基性イオン
酸・塩基
23.5K 閲覧数

15.11 : 塩水溶液のpHの決定
酸・塩基
43.3K 閲覧数

15.12 : 多価酸
酸・塩基
29.0K 閲覧数

15.13 : 酸性強度と分子構造
酸・塩基
30.7K 閲覧数

15.14 : ルイス酸及びルイス塩基
酸・塩基
43.4K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved