このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
組換えRCAS(A)レトロウイルスのニワトリ胚水晶体へのマイクロインジェクション
要約
このプロトコルペーパーはレンズの開発の間に蛋白質のその 場 機能そして表現を調査するための用具としてRCAS (A)のretrovirusの萌芽期の鶏レンズのmicroinjectionの方法論を記述する。
要約
胚ニワトリ(Gallus domesticus)は、ヒトの水晶体との類似性が高いことから、水晶体の発達と生理学の研究において確立された動物モデルです。RCAS(A)は、分裂細胞に感染する複製能力のあるニワトリレトロウイルスであり、発生初期に水晶体小胞の空腔にマイクロインジェクションすることにより、水晶体発生中の野生型および変異型タンパク質の in situ 発現と機能を研究するための強力なツールとして機能し、その作用を周囲の増殖する水晶体細胞に制限します。トランスジェニックモデルや ex vivo 培養などの他のアプローチと比較して、RCAS(A)複製能力のある鳥レトロウイルスの使用は、ニワトリ胚で外因性タンパク質を発現するための非常に効果的で迅速でカスタマイズ可能なシステムを提供します。具体的には、標的遺伝子導入は、組織特異的プロモーターを必要とせずに増殖性水晶体線維細胞に限定することができます。本稿では、組換えレトロウイルスRCAS(A)の調製に必要なステップを簡単に概説し、マイクロインジェクション手順の詳細かつ包括的な概要を提供し、この技術のサンプル結果を提供します。
概要
このプロトコルの目的は、RCAS(A)(複製有能な鳥肉腫/白血病レトロウイルスA)の萌芽期のニワトリ水晶体マイクロインジェクションの方法論を記述することです。ニワトリ胚の胎内レンズにおける効果的なレトロウイルス送達は、正常な水晶体の生理学、病理学的状態、および発生における水晶体タンパク質の分子メカニズムと構造機能の in vivo 研究のための有望なツールであることが実証されています。さらに、この実験モデルは、治療標的の同定や、ヒトの先天性白内障などの疾患の薬物スクリーニングに使用できます。全体として、このプロトコルは、レンズタンパク質の研究のためのカスタマイズ可能なプラットフォームの開発に必要な手順を説明することを目的としています。
胚のひよこ(Gallus domesticus)は、水晶体の構造と機能がヒトの水晶体と類似しているため、水晶体の発達と生理学の研究のための確立された動物モデルです1,2,3,4。RCAS(A)複製能力のある鳥レトロウイルスの使用は、ニワトリ胚で外因性タンパク質を発現するための非常に効果的で迅速でカスタマイズ可能なシステムと見なされています。特に、空のレンズ....
プロトコル
本研究は、動物福祉法および動物福祉実施規則を遵守し、「実験動物の飼育及び利用に関する手引き」の原則に従って実施された。すべての動物処置は、テキサス大学サンアントニオ校健康科学センターの動物飼育および使用委員会によって承認されました。プロトコルの概要については、 図 1 を参照してください。このプロトコルで使用されるすべての材料、試薬、および機器の詳細については、 材料表 を参照してください。
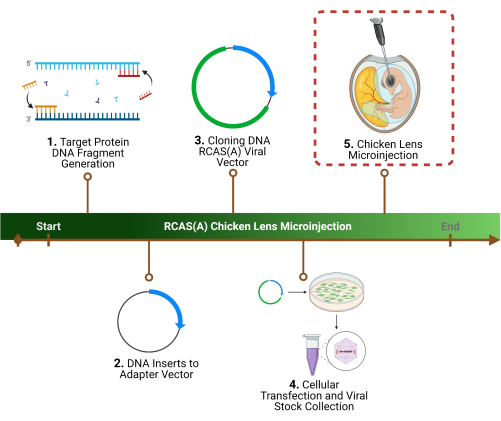
図1:実験概要1. プロトコルの最初のステップは準の遺伝子シーケンスの特定のターゲット蛋白質、同一証明、およびDNAのフラグメントの生成の決定です。 2 .アダプターベクターへの最初のクローニングによるレトロウイルスベクターへの遺伝子配列のクローニング、 3. その後にウイルスベクター。 4.....
代表的な結果
特定の標的タンパク質を決定し、関連する遺伝子配列を同定した後、全体的な実験アプローチでは、アダプターベクターへの最初のクローニング、続いてウイルスベクターへのクローニングにより、遺伝子配列をレトロウイルスRCAS(A)ベクターにクローニングします。次に、パッケージングセルを使用して高力価のウイルス粒子を調製し、ビリオンを回収して濃縮します。これらの最初の2つ?.......
ディスカッション
この実験モデルは、インタクトレンズに目的のタンパク質を発現させる機会を提供し、レンズの構造と機能におけるこれらのタンパク質の機能的関連性の研究につながります。胚性ニワトリマイクロインジェクションモデルは、Feketeらの研究に部分的に基づいています。al.6 と Jiang et によってさらに開発されました。また、ウイルスプラスミドと、アゴニ?.......
開示事項
著者らは、この研究は、潜在的な利益相反と解釈される可能性のある商業的または金銭的関係がない状態で実施されたと宣言しています。
謝辞
この研究は、米国国立衛生研究所(NIH)の助成金:RO1 EY012085(J.X.J.)およびF32DK134051(F.M.A.)、およびウェルチ財団の助成金:AQ-1507(J.X.J.)の支援を受けました。内容は著者の責任であり、必ずしも米国国立衛生研究所の公式見解を表すものではありません。フィギュアの一部は Biorender.com で作成されました。
....資料
| Name | Company | Catalog Number | Comments |
| 0.22 µm Filter | Corning | 431118 | For removing cellular debris from media |
| 35 mm x 10 mm Culture Dish | FisherScientific | 50-202-030 | For using during microinjection |
| Centrifuge | Fisherbrand | 13-100-676 | Spinning down solution |
| Constructs | GENEWIZ | - | For generation of constructs |
| Dissecting microscope | AmScope | SM-4TZ-144A | Visualization of lens for microinjection |
| DNA PCR primers | Integrated DNA Technologies | - | Generation of primers: Intracellular loop (IL)-deleted Cx50 (residues 1–97 and 149–400) as well as the Cla12NCO vector were obtained with the following pair of primers: sense, CTCCTGAGAACCTACATCCT; antisense, CACCGCATGCCCAAAGTACAC ILs of Cx43 (residues 98–150) and Cx46 (residues 98–166) were obtained with the following pairs of primers: sense, TACGTGATGAGGAAAGAAGAG; antisense, TCCTCCACGCATCTTTACCTTG; sense, CACATTGTACGCATGGAAGAG; antisense, AGCACCTCCC AT ACGGATTC, respectively Cla12NCO-Cx43 construct template was obtained with the following pair of primers: sense, CTGCTTCGTACTTACATCATC; antisense, GAACAC GTGCGCCAGGTAC ILs of Cx50 (residues 98–148) or Cx46 (residues 98–166) were cloned by using Cla12NCO-Cx50 and Cla12NCO-Cx46 constructs as the templates with the following pair of primers: sense, CACCATGTCCGCATGGAGGAGA; antisense, GGTCCCC TC CAGGCGAAAC; sense, CACATTGTACGCATGGAAGAG; antisense, AGCACCTCCCATACGGATTC, respectively |
| Drummond Nanoject II Automatic Nanoliter Injector | Drummond Scientific | 3-000-204 | Microinjection Pipet |
| Dual Gooseneck Lights Microscope Illuminator | AmScope | LED-50WY | Lighting for visualization |
| Dulbecco’s Modified Eagle Medium (DMEM) | Invitrogen | For cell culture | |
| Egg Holder | - | - | Homemade styrofoam rings with 2-inch diameter and one-half inch height |
| Egg Incubator | GQF Manufacturing Company Inc. | 1502 | For incubation of fertilized eggs |
| Fast Green | Fisher scientific | F99-10 | For visualization of viral stock injection |
| Fertilized white leghorn chicken eggs | Texas A&M University | N/A | Animal model of choice for microinjection (https://posc.tamu.edu/fertile-egg-orders/) |
| Fetal Bovine Serum (FBS) | Hyclone Laboratories | For cell culture | |
| Fluorescein-conjugated anti-mouse IgG | Jackson ImmunoResearch | 115-095-003 | For anti-FLAG 1:500 |
| Forceps | FisherScientific | 22-327379 | For moving things around and isolation |
| Glass capillaries | Sutter Instruments | B100-75-10 | Glass micropipette for microinjection (O.D. 1.0 mm, I.D. 0.75 mm, 10 cm length) |
| Lipofectamine | Invitrogen | L3000001 | For transfection |
| Manual vertical micropipette puller | Sutter Instruments | P-30 | To obtain glass micropipette of the correct size |
| Microcentrifuge Tubes | FisherScientific | 02-682-004 | Dissolving solution |
| Microscope | Keyence | BZ-X710 | For imaging staining |
| Parafilm | FisherScientific | 03-448-254 | Placing solution |
| Penicillin/Streptomycin | Invitrogen | For cell culture | |
| Pico-Injector | Harvard Apparatus | PLI-100 | For delivering small liquid volumes precisely through micropipettes by applying a regulated pressure for a digitally set period of time |
| rabbit anti-chick AQP0 | Self generated | - | Jiang JX, White TW, Goodenough DA, Paul DL. Molecular cloning and functional characterization of chick lens fiber connexin 45.6. Mol Biol Cell. 1994 Mar;5(3):363-73. doi: 10.1091/mbc.5.3.363. |
| rabbit anti-FLAG antibody | Rockland Immunichemicals | 600-401-383 | For staining FLAG |
| Rhodamine-conjugated anti-rabbit IgG | Jackson ImmunoResearch | 111-295-003 | For anti-AQP0 1:500 |
| Sponge clamping pad | Sutter Instruments | BX10 | For storage of glass micropipette |
参考文献
- Li, Z., Gu, S., Quan, Y., Varadaraj, K., Jiang, J. X. Development of a potent embryonic chick lens model for studying congenital cataracts in vivo. Communications Biology. 4 (1), 325 (2021).
- Chen, Y., et al.
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
JoVEについて
Copyright © 2023 MyJoVE Corporation. All rights reserved