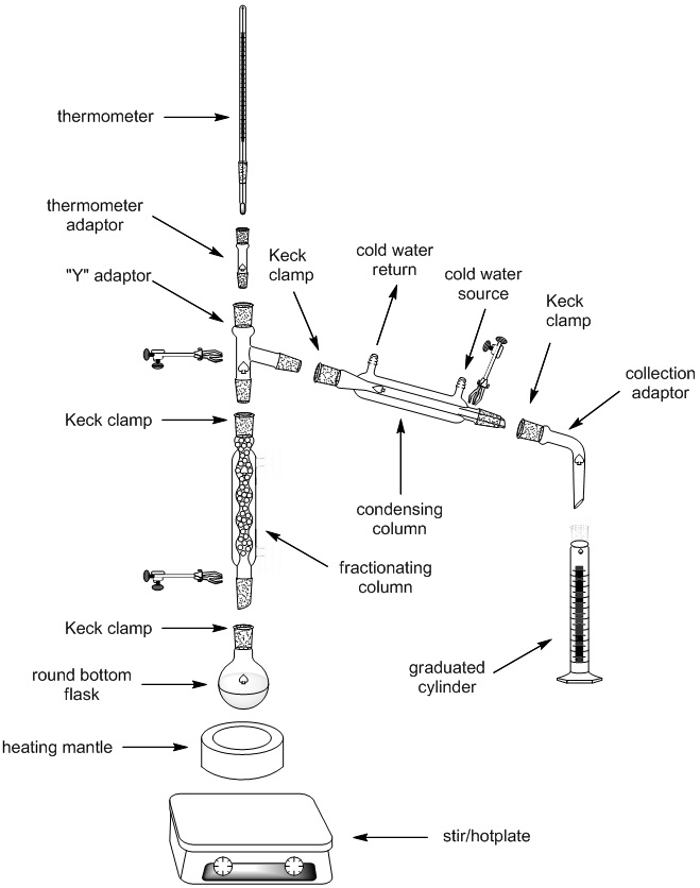
Fractional Distillation
Source: Laboratory of Dr. Nicholas Leadbeater — University of Connecticut
Distillation is perhaps the most common laboratory technique employed by chemists for the purification of organic liquids. Compounds in a mixture with different boiling points separate into individual components when the mixture is carefully distilled. The two main types of distillation are "simple distillation" and "fractional distillation", and both are widely used in organic chemistry laboratories.
Simple distillation is used when the liquid is (a) relatively pure (containing no more than 10% liquid contaminants), (b) has a non-volatile component, such as a solid contaminant, or (c) is mixed with another liquid with a boiling point that differs by at least 25 °C. Fractional distillation is used when separating mixtures of liquids whose boiling points are more similar (separated by less than 25 °C).
This video will detail the fractional distillation of a mixture of two common organic solvents, cyclohexane and toluene.
1. Set-up of Fractional Distillation Apparatus
| Fractional Distillation Apparatus | Quantity |
| Retort stands (ring stands) | 2 |
| Screw jack | 1 |
| Clamps | 4 |
| Keck clamps | 4 |
| Magnetic stir bar |