Removal of Branched and Cyclic Compounds by Urea Adduction for Uk'37 Paleothermometry
Source: Laboratory of Jeff Salacup - University of Massachusetts Amherst
As mentioned in previous videos, the product of an organic solvent extraction, a total lipid extract (TLE), is often a complex mixture of hundreds, if not thousands, of different compounds. The researcher is often only interested in a handful of compounds. In the case of our two organic paleothermometers (Uk'37 and MBT/CBT), the interest is in only 6 compounds (2 alkenones and 4 isoprenoidal glycerol dialkyl glycerol tetraethers). As discussed in the previous two videos in this series, purification techniques may be applied in order to pare down the number of compounds in an analyzed sample. These techniques may chemically alter the unwanted components (saponification), take advantage of the different compound chemistries (column chromatography), or use the different shapes and sizes of the molecules to include or exclude certain components from the analysis (urea adduction). The atomic structure of different chemicals leads some organic compounds to form long, narrow, straight chains (n-alkanes and alkenones), other organic compounds to form complex cyclic structures, others to form highly-branched structures, and yet others which form both cyclic and branched structures (GDGTs) (Figure 1). The different shapes and sizes of the compounds in a sample can be used to separate them from one another, in much the same way as a coin sorter separates coins of different denominations (sizes).
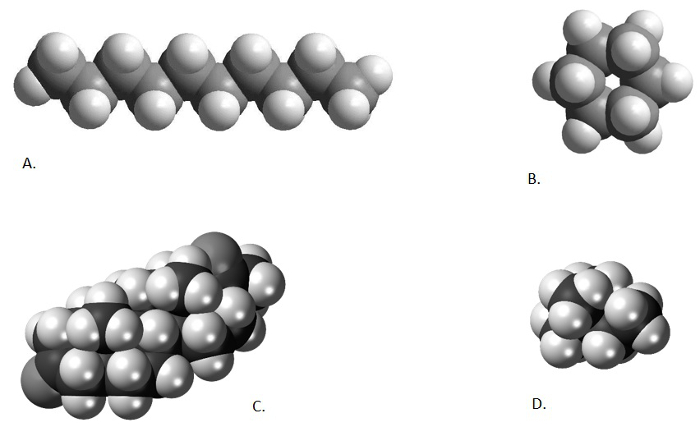
Figure 1. Comparison of different chemical structures. Decane, a straight-chained alkane (A; from from http://www.bpc.edu/mathscience/chemistry), cyclohexane, a cyclic alkane (B; from http://www.bpc.edu/mathscience/chemistry), a steroid, a poly-cyclic hydrocarbon (C; from www.wikiwand.com), and 2,2-dimethylbutane, a branched alkane (D; www.wikimedia.com). Please click here to view a larger version of this figure.
1. Setup and Preparation of Materials
- Obtain a total lipid extract (TLE) using a solvent extraction method (Sonication, Soxhlet, or Accelerated Solvent Extraction (ASE)).
- Purchase the following materials from any chemical retailer: combusted borosilicate glass pipettes and bulbs; pure water; hexane; Dichloromethane (DCM); methanol; urea.
- The reagents should be pure and free from hydrocarbons. Alternatively, pure water can be made in a lab using a water purification system.
This purification technique produces two different vials; one labeled adduct, containing straight-chained and rarely-branching compounds, and another labeled non-adduct, containing highly-branched and cyclic compounds. This procedure has vastly decreased the complexity of any sample to be analyzed on an instrument. This decrease in complexity is often crucial to the accurate analysis of target compounds. For example, in nearshore settings after approximately 1850, alkenones co-elute with
Urea adduction is often used in the purification of n-alkanes, common constituents of leaf wax, in order to remove co-eluting compounds before isotope analysis. The carbon and hydrogen isotope ratios of leaf waxes in plants contain information on the metabolic pathways and environmental conditions the plant used and lived in, respectively. In order to determine the isotope ratios, very large quantities of compound must be loaded onto a GC. Such large quantities often cause compounds that elute close to one another at low
건너뛰기...
이 컬렉션의 비디오:

Now Playing
Removal of Branched and Cyclic Compounds by Urea Adduction for Uk'37 Paleothermometry
Earth Science
6.3K Views

Determining Spatial Orientation of Rock Layers with the Brunton Compass
Earth Science
25.0K Views

Using Topographic Maps to Generate Topographic Profiles
Earth Science
31.5K Views

Making a Geologic Cross Section
Earth Science
46.4K Views

Physical Properties Of Minerals I: Crystals and Cleavage
Earth Science
51.3K Views

Physical Properties Of Minerals II: Polymineralic Analysis
Earth Science
37.6K Views

Igneous Volcanic Rock
Earth Science
39.2K Views

Igneous Intrusive Rock
Earth Science
32.2K Views

An Overview of bGDGT Biomarker Analysis for Paleoclimatology
Earth Science
5.4K Views

An Overview of Alkenone Biomarker Analysis for Paleothermometry
Earth Science
7.2K Views

Sonication Extraction of Lipid Biomarkers from Sediment
Earth Science
7.2K Views

Soxhlet Extraction of Lipid Biomarkers from Sediment
Earth Science
18.3K Views

Extraction of Biomarkers from Sediments - Accelerated Solvent Extraction
Earth Science
7.4K Views

Conversion of Fatty Acid Methyl Esters by Saponification for Uk'37 Paleothermometry
Earth Science
10.1K Views

Purification of a Total Lipid Extract with Column Chromatography
Earth Science
12.2K Views
JoVE 소개
Copyright © 2025 MyJoVE Corporation. 판권 소유