ELISPOT Assay: Detection of IFN-γ Secreting Splenocytes
Source: Tonya J. Webb1
1 Department of Microbiology and Immunology, University of Maryland School of Medicine and the Marlene and Stewart Greenebaum Comprehensive Cancer Center, Baltimore, Maryland 21201
ELISPOT is a standardized, reproducible assay used to detect cellular immune responses. The assay utilizes an enzyme-linked immunosorbent assay (ELISA)- based method to detect single-cell immune responses which can be visualized by spots, hence the name ELISPOT. ELISPOT was first described in 1983, by Czerkinsky, as a method of enumerating the number of B cell hybridomas producing antigen-specific immunoglobulins (1). The same group further developed the assay to measure the frequency of cytokine producing T lymphocytes. Now ELISPOT has become a gold standard for measuring antigen-specific T cell immunity in clinical trials and vaccine candidates. For example, after vaccination or during an infection, plasma cells and memory B cells secrete antibodies that provide protection. Typically, these B cell responses are assessed by measuring serum titers of antigen-specific antibodies. However, this type of analysis, typically measured by ELISA, may not include memory B cells, which can be present even in the absence of detectable serum antibody levels. Furthermore, it has been well-established that circulating memory B cells are important for the rapid and protective antibody response observed following pathogen re-exposure, thus it is critical to be able to detect these cells. Therefore, to clearly assess antigen-specific memory B-cell responses, both ELISA and ELISPOT should be used (2).
ELISPOT assay uses a plate containing membrane-lined wells that are coated with antibodies in order to capture secreted proteins of interest. Then, the plate is loaded with cells and stimuli to induce protein production. The secreted proteins are captured by the antibodies coated on the surface. After appropriate incubation time, cells are removed and the secreted molecule is detected by using a biotinylated antibody that is specific for a different epitope, as compared to the capture antibody. Next, streptavidin peroxidase is added, followed by the addition of a substrate that allows the detection of the spots (Figure 1). The strength of this assay is that it allows one to quantitate the number of cells producing the protein of interest. Importantly, one can assess if there are changes in the total number of cells producing a specific protein or if individual cells within a population are producing more protein. Moreover, it can provide information regarding kinetics and can be used to assess overall immune activation (mitogen stimulation) relative to antigen-specific responses (antigen simulation). The ELISPOT assay will permit the detection one activated cell amongst 300,000 cells following mitogenic or antigen-specific activation.
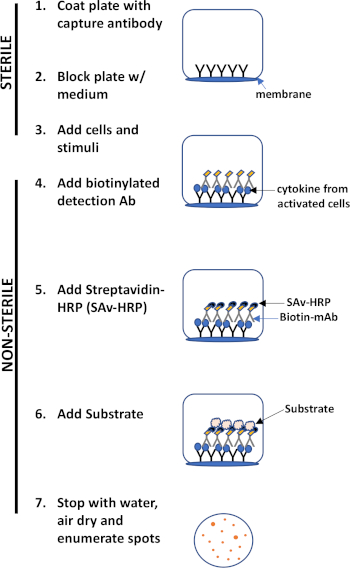
Figure 1: ELISPOT protocol overview.
The major advantages of this assay are its- a. Simplicity- the protocol is relatively simple and straightforward. It does not require technical expertise, b. Sensitivity- it permits the detection of immune cells at the single cell level and requires very few cells compared to other methods such as flow cytometry, c. Functionality- it provides quantitative data regarding immune function.
This lab exercise demonstrates the ELISPOT protocol for detection of IFN-γ secreting-splenocytes, but as mentioned above this assay can also be used to assess antibody secretion by B cells (3).
1. Set-up
Buffers and Reagents
- Sterile phosphate-buffered saline (PBS) without calcium or magnesium
- Coating buffer- either sterile PBS or carbonate buffer
- Assay diluent- 10% fetal bovine serum (FBS) in PBS
- Cell culture medium- RPMI 1640 with 10% FBS, penicillin/streptomycin, & L-glutamine
- Wash buffer- PBS containing 0.05% Tween20
- Double distilled water (ddH2O)
- Detection subs
In this ELISPOT assay, splenic leukocytes from wildtype and tumor-bearing mice were analyzed for IFN-γ. Figure 2 A shows the visual image of the assay result. The numbers in the green color indicate the number of spots per well (TNTC indicates "too numerous to count"). Notice that the number of spots decreases with decreasing cell concentration.
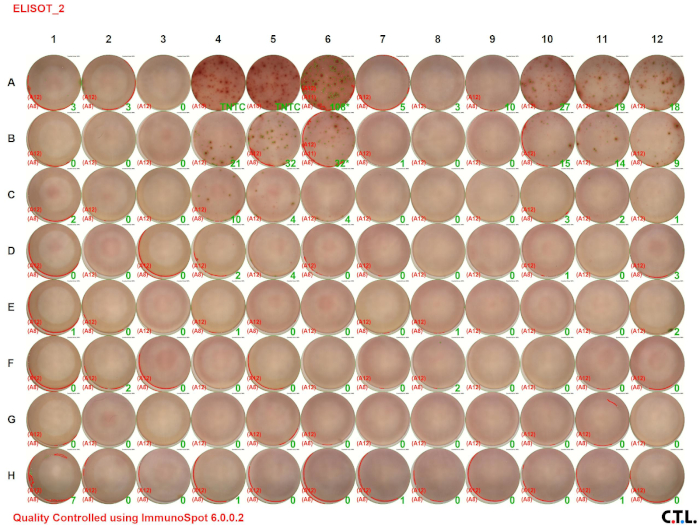
The ELISPOT assay allows one to assess immune cell activation by determining the number of cells secreting a specific analyte. The size and intensity of the spots provides information regarding the amount of analyte being produced by each cell. The protocol outlined above detailed the detection of a single cytokine. However, recent developments have enhanced the utility of this assay. Currently, one can use fluorescent detection dyes in order to detect multiple analytes within a well. This permits the detection of differ
- Czerkinsky, C. C., Nilsson, L. A., Nygren, H., Ouchterlony, O., & Tarkowski, A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. Journal of Immunological Methods, 65 (1), 109-121(1983).
- Wahid, R., Simon, J. K., Picking, W. L., Kotloff, K. L., Levine, M. M., & Sztein, M. B. Shigella antigen-specific B memory cells are associated with decreased disease severity in subjects challenged with wild-type Shigella flexneri 2a. Clinical Immunology, 148 (1), 35-43 (2013).
- Roberts, T. J., Lin, Y., Spence, P. M., Van Kaer, L., & Brutkiewicz, R. R. CD1d1-dependent control of the magnitude of an acute antiviral immune response. The Journal of Immunology, 172, 3454-3461 (2004).
건너뛰기...
JoVE 소개
Copyright © 2024 MyJoVE Corporation. 판권 소유