Identification of EcoHIV-Infected Cells in Microglia-Manipulated Transgenic Mice
In This Article
Summary
This protocol describes how the combination of EcoHIV infection with Tmem119-EGFP mice offers a valuable biological system for investigating microglial alterations and viral reservoirs in rodent models of HIV-associated neurocognitive disorders.
Abstract
Combined antiretroviral therapy (cART) has dramatically improved the quality of life for people living with HIV (PLWH). However, over 4 million PLWH are over the age of fifty and experience accompanying HIV-associated neurocognitive disorders (HAND). To understand how HIV impacts the central nervous system, a reliable and feasible model of HIV is necessary. Previously, a novel biological system using chimeric HIV (EcoHIV) inoculation was developed in a rat model to investigate neurocognitive impairments and synaptic dysfunction. Nevertheless, a significant challenge remains in clarifying EcoHIV's neuroanatomical distribution, particularly its differential expression in various cell types in the brain. In the current study, EcoHIV with mScarlet fluorescence labeling was modified and retro-orbitally injected into Tmem119-EGFP knock-in mice (which express enhanced green fluorescence protein primarily in microglia) to determine if microglia are the major cell type responsible for viral expression and reservoirs of HIV in the brain. The current data show that: (1) in vitro, EcoHIV-mScarlet fluorescence signals were predominantly localized in microglia-like cells among primary rodent brain cells; (2) in vivo, injection of EcoHIV-mScarlet into Tmem119-EGFP mice induced significant HIV expression in the mouse brain. The co-localization of mScarlet and EGFP signals suggests that microglia are the main cell type harboring HIV in the brain. Overall, EcoHIV in rodents offers a valuable biological system to study microglial alterations, viral reservoirs in the brain, and the neurological mechanisms of HIV-associated neurocognitive disorders.
Introduction
Despite the profound benefits derived from antiretroviral therapy, people living with HIV (PLWH) still experience neurocognitive disorders. To better understand the neuronal mechanisms of HIV-associated neurocognitive disorder (HAND), there is a critical need for HIV models to further elucidate the specific cell type involvement in NeuroHIV.
The HIV-1 transgenic rat, which has constitutive exposure to HIV-1 viral proteins, is a popular rodent model used to investigate neurocognitive disorders1,2,3,4and neuroanatomical alterations5,6,7 associated with HAND. The functional deletion of the gag and pol domains prevents viral replication, rendering the HIV-1 transgenic rat noninfectious8,9. Recently, a chimeric HIV (EcoHIV) infection model in mice was initially reported by Potash et al.10 and later extended to rats, which may be advantageous for further studies on HAND and substance use disorders11. In this novel biological system, systemic HIV-1 infection was observed along with many clinical features of HIV-1 in humans, including lymphocyte and macrophage involvement, antiviral immune responses, neuroinvasiveness, and brain inflammation.
Microglia play a critical role as specialized brain-resident macrophages in maintaining brain function and homeostasis. To distinguish microglia from closely related cell types (e.g., blood monocytes, perivascular macrophages, meningeal macrophages), the current study used the Tmem119-EGFP knock-in mouse line. Previous studies have reported that transmembrane protein 119 (Tmem119) exhibits an exclusively microglia-specific expression pattern in rodent and human brain tissue12,13,14,15. The EGFP signal in Tmem119-EGFP knock-in mice was observed throughout the brain and localized specifically to microglial cells.
In the present study, Tmem119-EGFP knock-in mice were inoculated with the EcoHIV-mScarlet virus, and cells positive for EcoHIV in the central nervous system were identified. Here, we present a protocol for EcoHIV-mScarlet inoculation in Tmem119-EGFP knock-in mice, providing a reliable model for therapeutically targeting microglial alterations in HIV.
Protocol
The Animal Care and Use Committee at the University of South Carolina approved all animal protocols (federal assurance number: D16-00028). All experiments strictly followed the guidelines established by the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals. Tmem119-EGFP knock-in mice (30 days old, male, 23-26 g body weight) were obtained from a commercial source and group-housed in AAALAC-accredited facilities. All animals were housed under a 12/12 h light-dark cycle with free access to food and water. The details of the animals, reagents, and equipment used in this study are listed in the Table of Materials.
1. EcoHIV-mScarlet packaging in 293FT cells
- Incubate the 293 FT cells in a gelatin-precoated 75 cm2 flask. Keep cells growing to 30% confluent at transfection.
- Perform the transfection of plasmid DNA (15 µg) of EcoHIV-mScarlet (Supplementary File 1) using Lipofectamine 3000 (22.5 µL) following the manufacturer's instructions (see Table of Materials).
- Culture the cells in DMEM medium with 10% FBS serum for 3 days at 37 °C.
- Collect the conditional medium with viral suspension. Centrifuge at 500 × g for 10 min at 4 °C. Transfer the supernatant with a 10 mL pipette to a sterile 50 mL tube.
- Add a certain amount of Lenti-x concentrator (1:3 ratio) into the viral mixture (for example, 8 mL of concentrator with 24 mL of viral mixture). Gently invert the tube five times.
- Keep the virus-Lenti concentrator mixture at 4 °C for 2 days. Centrifuge at 1,500 × g, 45 min, 4 °C. Carefully remove supernatant as much as possible using a pipette.
- Re-suspend the pellet with prechilled 200 µL of 100 mM PBS. Store the virus at -80 °C.
NOTE: The details of EcoHIV-mScarlet packaging in 293FT cells were described in our previous study16. Do not vortex or introduce air bubbles in the viral solution.
2. EcoHIV-mScarlet infection in primary rat brain cells
- Perform the primary brain cell isolation from rat fetuses (18 days) following the previously published report16.
- Transfer dissociated cells to poly-L-Lysine pre-coated 12-well plates with glass inserts containing 1 mL of DMEM/F12 medium plus 10% FBS. Replace medium the next day for Neurobasal medium with B27 supplement.
- Culture primary fetal brain cells in a 5% CO2 incubator for 3 weeks.
- Add EcoHIV-mScarlet (60 µL, 1.26 × 106 TU/mL) into the culture medium. Incubate the cultured brain cells with EcoHIV-mScarlet for 6 days.
- Fix the cells with 4% PFA and perform immunostaining on infected brain cells using specific primary antibodies (CD11b/c, Iba1).
- Obtain images using 40× objective under a confocal microscopy system.
3. EcoHIV-mScarlet virus infection in primary glia cells from adult mice
- Anesthetize adult mice with 5% sevoflurane for 5 min (following institutionally approved protocols). Sterilize the skin of the head with 70% EtOH.
- After confirming the mouse is no longer responsive to noxious stimuli, use a sterilized sharpened scissor to perform decapitation. Transfer the head to a new Petri dish filled with 5 mL of HBSS.
- Open the scalp and transfer brain tissue into another Petri dish containing 5 mL of sterilized HBSS. Peel off the meninges and transfer the frontal cortex into 2 mL of HBSS.
- Add 20 µL of 0.25% Trypsin-EDTA into the mixture. Incubate for 15 min at room temperature; gently swirl the tube every few minutes.
- Transfer dissociated cells to a poly-L-Lysine pre-coated 75 cm2 flask containing 10 mL of DMEM/F12 medium and 10% FBS.
- Culture cells at 37 °C, 5% CO2 incubator, until 90% confluency. Digest brain cells with 2 mL of 0.25% Trypsin-EDTA.
- Subculture brain cells into 35 mm glass-bottom dishes containing 5 mL of DMEM/F12 growth medium till 80% confluency.
- Add EcoHIV-mScarlet (8 µL, 1.26 × 106 TU/mL) into the culture medium. Incubate mouse glial cells for 2 days.
- Check red (mScarlet) fluorescence signals daily under a fluorescent microscope.
4. EcoHIV-mScarlet virus retroorbital injection into Tmem119-EGFP mice
- Use 3% sevoflurane to anesthetize the Tmem119-EGFP mice (both male and female mice at 30 days of age) until they are no longer responsive to noxious stimuli.
- Secure the mice in a lateral position with the injection eye facing up and respiration through the nose cone, which is linked to an anesthesia system. Use an appropriate nose cone size to provide continuous anesthesia.
- Thaw the EcoHIV-mScarlet on ice. Fill the viral solution into an intraocular injector syringe with a 33 G blunt needle.
- Place the mouse in the right lateral recumbency and keep its head facing to the left. Identify the location of the medial canthus as the injection site.
- After proptosing the eye, slowly and gently insert a needle (45-degree angle) into the medial canthus of the eye. Carefully insert the needle forward into the vessels behind the eyeball (retro-orbital sinus).
- Gently inject 6.5 µL of EcoHIV-mScarlet (1.26 × 106 TU/mL, bilateral-eye inoculation) into the retro-orbital sinus. Carefully remove the needle from the retro-orbital sinus and gently apply pressure to the eyelids to provide hemostasis.
- Apply lubricant to the eye to prevent the cornea from drying or becoming injured.
- Allow the mice to recover in a recovery chamber with a heating pad until it has woken up.
NOTE: The needle bevel should not be positioned too deep so that the arteries are not ruptured or the bones fractured. Viral infusion time is dependent upon multiple factors (e.g., injection volume, titer, animal size). For EcoHIV viral infusion, significant expression has been observed one week after retro-orbital injections11,16,17.
5. Visualization of brain tissue slices
- Deeply anesthetize mice using 5% sevoflurane. Move on to step 5.2 when the mice show no response to noxious stimuli and reflexes are absent.
- Keep the mice in a supine position inside a chemical fume hood.
- Open the skin along the thoracic midline. Separate the diaphragm and open the chest with scissors.
- Insert a 22 G1 1/2 needle into the left ventricle. Open the right atrium with scissors.
- Perfuse 50 mL of pre-chilled 100 mM PBS. Perfuse 100 mL of pre-chilled 4% paraformaldehyde buffer16.
- Remove the entire mouse brain16.
- Fix overnight with 4% paraformaldehyde.
- Secure the brain tissue using tissue adhesive on the metal platform of the Vibratome. Cut 50 µm thickness of coronal sections with carbon steel blades.
- Place the brain slices onto glass slides using a brush. Immediately add 0.1 mL of the antifade mounting medium to each section.
- Place a 22 mm x 50 mm coverslip over brain sections. Dry the super-frost slides in the dark for 1 day.
- Configure the confocal microscope system to a magnification of 60× (A/1.4, oil) and set a Z-plane interval of 0.15 µm, with a pinhole size of 30 µm and a back-projected pinhole radius of 167 nm.
- Use the 488 nm and 594 nm wavelengths to acquire multi-channel images of brain regions of interest.
Representative Results
A fragment of mScarlet (1858 bp) containing enzyme sites of "Cla1" at the 3' end and "Not1" at the 5' end was inserted into pNL4-3-EcoHIV lentiviral vector (Figure 1). To validate the expression of EcoHIV-mScarlet, primary brain cells isolated from the cortex of rat E18 embryos were treated with EcoHIV-mScarlet (60 µL, 1.26 × 106 TU/mL) for 6 days in vitro. The data in Figure 2 showed that red fluorescent signals of mScarlet were mainly localized in glial types of cells based on the different cell morphology. Furthermore, CD11b/c and Iba1 (cell markers for microglia) labeling showed that mScarlet signals were co-localized with CD11b/c + and/or Iba1 + cells. The data indicated that microglia were the major cell type of EcoHIV-mScarlet distribution in vitro. There was no significant neuronal infection in the cultured cells (Supplementary Figure 1).
Next, the infection of EcoHIV-mScarlet was tested on adult mouse primary mixed glial cells. To do so, mixed glia cells were first isolated and purified from adult mice (2 months) and infected with EcoHIV-mScarlet (8 µL, 1.26 × 106 TU/mL) for 2 days. The images in Figure 3 showed that EcoHIV-mScarlet successfully infected adult mouse glia.
To further address the distribution pattern of EcoHIV-mScarlet in the mouse brain, specifically for identifying the infected cell type, EcoHIV-mScarlet was retro-orbital injected into Tmem119-EGFP knock-in mouse line in which microglia were specifically labeled with EGFP signals without any other macrophage types of conflicts5. The results in Figure 4 (also in Supplementary Figure 2) show that mScarlet red fluorescence signals were observed in EGFP-positive cells, suggesting microglia as the major cell type of EcoHIV expression in mouse brains.
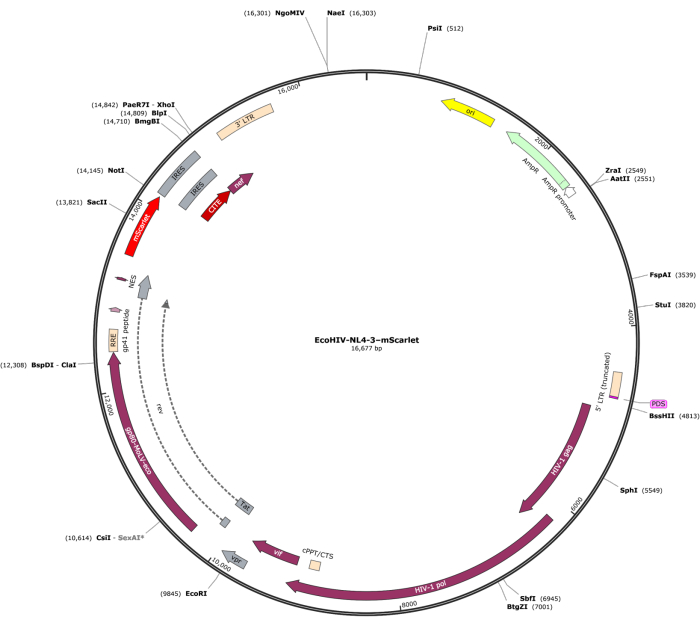
Figure 1: The vector map of EcoHIV-NL-4-3-mScarlet. Please click here to view a larger version of this figure.
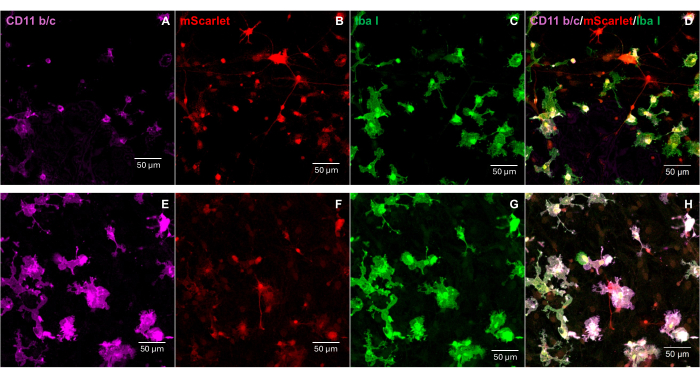
Figure 2: The EcoHIV-mScarlet infection in primary rat brain cells. (A,E) Representative images of CD11b/c staining at primary brain cells. The brain cells were isolated from E18 rat embryos and infected with the EcoHIV-mScarlet virus for 6 days. (B,F) Representative images of mScarlet fluorescent signals from in vitro brain cells. (C,G) Representative images of Iba1 staining at primary brain cells. (D,H) Merged images of triple labeling of CD11b/c, mScarlet, and Iba1. Scale bars: 50 µm. Please click here to view a larger version of this figure.
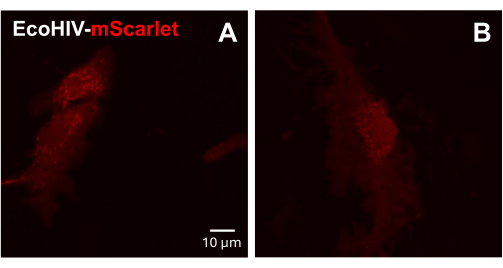
Figure 3: EcoHIV-mScarlet infection in primary mouse mixed glial cells. (A,B) Representative confocal images of mScarlet distribution in vitro. The primary mixed glia cells were isolated from adult C57BL6 mice (2-month-old) and cultured for 2 weeks before viral infection. The EcoHIV-mScarlet was added into the culture medium for two days and captured the images under the 60× objective of the Confocal microscope. Scale bar: 10 µm. Please click here to view a larger version of this figure.
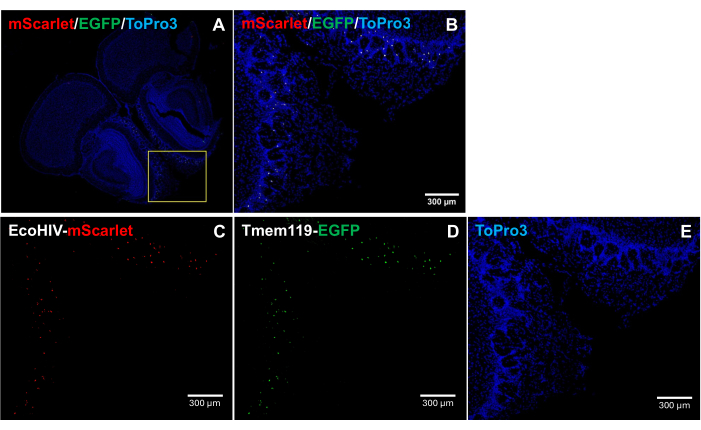
Figure 4: EcoHIV-mScarlet distribution in Tmem119-EGFP knock-in mouse line. (A,B) Merged images of mScarlet/EGFP/ToPro3 signals in brain sections. The yellow frame indicates the target area of (B). Scale bar: 300 µm. (C) Representative image of mScarlet distribution in the external plexiform layer of the olfactory region of Tmem119-EGFP knock-in mouse line. (D) Representative image of EGFP distribution. The fluorescent signals were localized in microglia cells in the Tmem119-EGFP knock-in mouse line. (E) Representative image of TO-PRO-3 nucleus staining. Scale bars: 300 µm. Please click here to view a larger version of this figure.
Supplementary Figure 1: MAP2 and MOG staining of primary rat brain cells infected with EcoHIV-mScarlet virus. Scale bars: 50 µm. Please click here to download this File.
Supplementary Figure 2: Confocal images of EcoHIV-mScarlet infection in Tmem119-EGFP knock-in mouse line. Scale bars: 75 µm. Please click here to download this File.
Supplementary File 1: EcoHIV-mScarlet plasmid DNA sequence. Please click here to download this File.
Discussion
In the present study, it was found that (1) the novel EcoHIV-mScarlet successfully infected primary rat brain cells in vitro; (2) the triple labeling of mScarlet, CD11b/c, and Iba1 identified microglia as the predominant cell type for this EcoHIV expression in rat brain cells in vitro; (3) primary mouse brain cells from adults in vitro further evidence the EcoHIV-mScarlet infection; (4) the EcoHIV-mScarlet distribution in the Tmem119-EGFP knock-in mouse line demonstrated a microglia-specific distribution pattern of EcoHIV infection.
Emerging studies suggested that various types of brain cells identified within the central nervous system (neurons, astrocytes, microglia, oligodendrocytes, etc.) exhibit functional and transcriptomic alteration during HIV and HIV-associated neurocognitive disorders18,19. For example, astrocytes contribute 30%-70% of the brain and perform surveillance to maintain brain homeostasis20. Astrocytes also modulate immune function and regulate the secretion of multi-cytokines and chemokines, especially in the situation of brain inflammation and neurodegeneration in HIV21,22. HIV infection of microglia not only results in a continuous release of viral proteins and proinflammatory cytokines and chemokines, but also provides predominant sources for HIV viral reservoirs23,24,25,26. Furthermore, activated microglia contribute a critical immunological function in the CNS; however, prolonged activation may also exacerbate neurodegeneration in HIV progression10,27. Oligodendrocytes also play an important function, including releasing several neurotrophins (such as nerve growth factor, brain-derived neurotrophic factor, etc.)28. A previous study found that the number of oligodendrocytes significantly decreased in the brains of AIDS patients, which may indicate direct damage to oligodendrocytes from HIV viral proteins29. Therefore, a specific type of cell-manipulated infection model of HIV should provide fundamental means of identifying the differential functions of various brain cells following infection. In the current study, a biological system was developed to mimic features of HIV-1 by chimeric HIV (EcoHIV) inoculation. This HIV inoculation was also combined with the Tmem119-EGFP knock-in mouse line to generate and validate a microglia-manipulated rodent model of HIV.
However, the limitations of the present study must be recognized. There were a few Iba1/CD11b/c negative cells that presented mScarlet fluorescent signals in vitro. Other types of brain cells, such as brain macrophages or pericytes, may be involved in EcoHIV infection, or alternatively, the cell culture environment may promote aberrant infection patterns relative to in vivo inoculation. Future, whole animal studies should further clarify the function of mScarlet+ microglia in the process of EcoHIV infection, and further define the regional distribution of mScarlet+EcoHIV microglia in the brain. Additionally, neurocognitive alterations occurring as a result of microglial infections may also be addressed in this rodent model. Collectively, the EcoHIV-mScarlet inoculation of Tmem119-EGFP knock-in mice provides a novel model and research strategy for investigating the microglial-driven mechanisms of HIV-associated neurocognitive disorders.
Disclosures
None of the authors have conflicts of interest to declare.
Acknowledgements
This work was funded by NIH grants DA059310, DA058586, AG082539, and GM109091. We appreciate the generous gift of the EcoHIV-NL4-3-EGFP from Dr. Potash of the Icahn School of Medicine at Mount Sinai.
Materials
| Name | Company | Catalog Number | Comments |
| 293FT cells | ThermoFisher Scientific | R70007 | |
| 33 G, Small Hub RN Needle, (Point Style: 3, Needle Length: 19.25 mm) | Hamilton | 7803-05 | |
| Antibiotic-Antimycotic solution | Cellgro | 30004CI | 100x |
| C57BL/6-Tmem119em2Gfng/J | The Jackson Laboratory | Strain #:031823 | |
| Corning BioCoat Gelatin 75cm² Rectangular Canted Neck Cell Culture Flask with Vented Cap | Life Technologies | 354488 | |
| Corning DMEM with L-Glutamine, 4.5 g/L Glucose and Sodium Pyruvate | Life Technologies | 10013CV | |
| Cover glass | VWR | 637-137 | |
| Dumont #5 Forceps | World Precision Instruments | 14095 | |
| Dumont #7 Forceps | World Precision Instruments | 14097 | |
| EndoFree Plasmid Maxi Kit (10) | Qiagen | 12362 | |
| Eppendorf Snap-Cap Microcentrifuge Biopur Safe-Lock Tubes | Life Technologies | 22600028 | |
| Feather Double Edge Carbon Steel Blades | Ted Pella, inc. | 121-9 | |
| Fisher BioReagents Microbiology Media: LB Broth, Miller | Fisher Scientific | BP1426-500 | |
| Human recombinant anti-CD11b antibody | Miltenyi Biotec | 130-120-214 | 1:50 dilution |
| Intraocular Injector Syringe (6.5 µL), Removable Needle | Hamilton | 6609071-01 | |
| Invitrogen Lipofectamine 3000 Transfection Reagent | Life Technologies | L3000015 | |
| Invitrogen One Shot TOP10 Electrocomp E. coli | Fisher Scientific | C404052 | |
| Iris Forceps | World Precision Instruments | 15914 | |
| Iris Scissors | World Precision Instruments | 500216 | |
| Lentivirus-Associated p24 ELISA Kit | Cell Biolabs, inc. | VPK-107-5 | |
| Lenti-X Concentrator | Takara | PT4421-2 | |
| Opti-MEM I Reduced Serum Medium | Life Technologies | 11058021 | |
| Paraformaldehyde | Sigma-Aldrich | 158127-500G | |
| Paraformaldehyde | Sigma | P6148 | |
| PELCO easiSlicer Vibratory Tissue Slicer | Ted Pella, inc. | 11000 | |
| PELCO Pro CA44 Tissue Adhesive | Ted Pella, inc. | 10033 | |
| PELCO Pro Specimen Retrievers | Ted Pella, inc. | 101-33 | |
| ProLong Gold | Fisher Scientific | P36930 | |
| Rabbit monoclonal anti-Iba1 antibody | Abcam | ab178847 | 1:500 dilution |
| RN Compression Fitting 1 mm | Hamilton | 55750-01 | |
| Sevoflurane | Merritt Veterinary Supply | 347075 | |
| Sprague Dawley pregnant rat | Inotiv | ||
| SuperFrost Plus Slides | Fisher Scientific | 12-550-154% | |
| To-Pro-3 | ThermoFisher Scientific | T3605 | Nucleus staining kit |
| Trypsin-EDTA (0.25%), phenol red | ThermoFisher Scientific | 25200-056 | |
| Vannas Scissors | World Precision Instruments | 500086 |
References
- Vigorito, M., Lashomb, A. L., Chang, S. L. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2, 319-328 (2007).
- Moran, L. M., Booze, R. M., Mactutus, C. F. Time and time again: Temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J. Neuroimmune Pharmacol. 8 (4), 988-997 (2013).
- Repunte-Canonigo, , et al. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol Neurodegener. 9, 26 (2014).
- Reid, W., et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA. 98 (16), 9271-9276 (2001).
- McLaurin, K. A., Li, H., Booze, R. M., Mactutus, C. F. Disruption of timing: NeuroHIV progression in the post-cART era. Sci Rep. 9, 827 (2019).
- Roscoe, R. F., Mactutus, C. F., Booze, R. M. HIV-1 transgenic female rat: Synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol. 9 (5), 642-653 (2014).
- Denton, A. R., et al. Selective monoaminergic and histaminergic circuit dysregulation following long-term HIV-1 protein exposure. J NeuroVirol. 25 (4), 540-550 (2019).
- Peng, J., et al. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 218 (1-2), 94-101 (2010).
- Abbondanzo, S. J., Chang, S. L. HIV-1 transgenic rats display alterations in immunophenotype and cellular responses associated with aging. PLoS ONE. 9, e105256 (2014).
- Potash, M. J., et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci USA. 102 (10), 3760-3765 (2005).
- Li, H., McLaurin, K. A., Illenberger, J. M., Mactutus, C. F., Booze, R. M. Microglial HIV-1 expression: Role in HIV-1 associated neurocognitive disorders. Viruses. 13 (5), 924 (2021).
- Kaiser, T., Feng, G. Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro. 6 (4), (2019).
- Bennett, M. L., et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci. USA. 113 (12), E1738-E1746 (2016).
- Satoh, J., et al. TMEM119 marks a subset of microglia in the human brain. Neuropathology. 36 (1), 39-49 (2016).
- Li, H., Aksenova, M., Bertrand, S. J., Mactutus, C. F., Booze, R. M. Quantification of filamentous actin (F-actin) puncta in rat cortical neurons. J Vis Exp. (108), e53697 (2016).
- Li, H., McLaurin, K. A., Mactutus, C. F., Booze, R. M. A rat model of EcoHIV brain infection. J Vis Exp. (167), e62137 (2021).
- Alfar, H. R., et al. Protocol for optimizing production and quality control of infective EcoHIV virions. STAR Protoc. 4 (3), 102368 (2023).
- Malatesta, P., Hartfuss, E., Götz, M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 127 (24), 5253-5263 (2000).
- Parpura, V., et al. Glutamate-mediated astrocyte-neuron signaling. Nature. 369 (6483), 744-747 (1994).
- Wahl, A., Al-Harthi, L. HIV infection of non-classical cells in the brain. Retrovirology. 20 (1), 1 (2023).
- Pandey, H. S., Seth, P. Friends turn foe-astrocytes contribute to neuronal damage in NeuroAIDS. J. Mol. Neurosci. 69 (2), 286-297 (2019).
- Minagar, A., et al. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer's disease, and multiple sclerosis. J Neurol Sci. 202 (1-2), 13-23 (2002).
- Borrajo, A., Spuch, C., Penedo, M. A., Olivares, J. M., Agís-Balboa, R. C. Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis. Ann Med. 53 (1), 43-69 (2021).
- González-Scarano, F., Martín-García, J. The neuropathogenesis of AIDS. Nat Rev Immunol. 5 (1), 69-81 (2005).
- Li, H., McLaurin, K. A., Mactutus, C. F., Booze, R. M. Microglia proliferation underlies synaptic dysfunction in the prefrontal cortex: Implications for the pathogenesis of HIV-1-associated neurocognitive and affective alterations. J. Neurovirol. 29 (4), 460-471 (2023).
- Kim, B. H., et al. EcoHIV infection of primary murine brain cell cultures to model HIV replication and neuropathogenesis. Viruses. 16 (5), 693 (2024).
- Réu, P., et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 20 (4), 779-784 (2017).
- Jones, J. D. Potential of glial cell modulators in the management of substance use disorders. CNS Drugs. 34 (7), 697-722 (2020).
- Kaalund, S. S., Johansen, A., Fabricius, K., Pakkenberg, B. Untreated patients dying with aids have loss of neocortical neurons and glial cells. Front Neurosci. 13, 1398 (2020).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved