Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Generation of Human CD40-activated B cells
Podsumowanie
In this video we present the ex vivo generation and expansion of human CD40-activated B cells (CD40-B) from peripheral blood mononuclear cells (PBMC) by stimulation with CD40 ligand and interleukin-4.
Streszczenie
CD40-activated B cells (CD40-B cells) have been identified as an alternative source of immuno-stimulatory antigen-presenting cells (APC) for cancer immunotherapy 1-3. Compared to Dendritic cells (DCs), the best characterized APC, CD40-B cells have several distinct biological and technical properties. Similar to DCs, B cells show an increased expression of MHC and co-stimulatory molecules (Fig.1b), exhibit a strong migratory capacity and present antigen presentation efficiently to T cells, after stimulation with interleukin-4 and CD40 ligand (CD40L). However, in contrast to immature or mature DCs, CD40-B cells express the full lymph node homing triad consisting of CD62L, CCR7/CXCR4, and leukocyte function antigen-1 (LFA1, CD11a/CD18), necessary for homing to secondary lymphoid organs (Fig.1a) 3. CD40-B cells can be generated without difficulties from very small amounts of peripheral blood which can be further expanded in vitro to very large amounts of highly-pure CD40-B cells (>109 cells per patient) from healthy donors as well as cancer patients (Fig.1c,d) 1,4.
In this protocol we demonstrate how to obtain fully activated CD40-B cells from human PBMC. Key molecules for the cell culture are CD40 ligand, interleukin-4 (IL-4) and cyclosporin A (CsA), which are replenished in a 3-4 day culture cycle. For laboratory purposes CD40-stimulation is provided by NIH/3T3 cells expressing recombinant human CD40 ligand (tCD40L NIH/3T3) 5. To avoid contamination with non-transfected cells, expression of the human CD40 ligand on the transfectants has to be checked regularly (Fig.2).
After 14 days CD40-B cell cultures consist of more than 95% pure B cells and an expansion of CD40-B cells over 65 days is frequently possible without any loss of function 1, 4. CD40-B cells efficiently take up, process and present antigens to T cells 6. They do not only prime naϊve, but also expand memory T cells 7,8. CD40-activated B cells can be used to study B-cell activation, differentiation and function. Moreover, they represent a promising tool for therapeutic or preventive vaccination against tumors 9.
Protokół
The protocol for the generation of human CD40-activated B cells from PBMC is divided into two parts: Part A demonstrates the preparation of CD40 ligand expressing NIH/3T3 cells, which will be used as plate-bound feeder cells. Part B describes the actual CD40-B culture.
A. Preparation of feeder cells (tCD40L NIH/3T3)
The tCD40L NIH/3T3 is an adherent murine fibroblast cell line, which should never become completely confluent. The cells are therefore splitted twice per week. Culturing over more than 6 weeks is not recommended.
- Remove old medium from the primary culture with a sterile pipette and wash cells with 10 mL of 1x PBS. Aspirate the PBS after washing.
- Add 4 mL Trypsin/EDTA in a 75 cm2 flask for 5-10 minutes at 37°C. Use gentle tapping to detach the cells.
- Add 10 mL of wild type medium and swivel gently.
- Transfer the cell suspension into a 50 mL tube with a sterile pipette and spin the cells down at 225 x g for 5 min.
- Remove the supernatant and resuspend the pellet in 10 mL of wild type medium. Count the cell number of an aliquot of the cell suspension and prepare three 50 mL tubes with the appropriate number of cells:
- 1.5 x 106 cells for subculturing
- 0.2 x 106 cells/well for irradiation used for the CD40-B cell culture
- remainder to freeze (if needed).
- Spin the cells down at 225 x g for 5 min.
- Remove the supernatant.
- For subculturing: resuspend 1.5 x 106 cells in 10 mL wild type medium in a 75 cm2 cell culture flask (cell density 1.5 x 105 cells/mL), add G-418 [0.7 mg/mL] and incubate the cells at 37°C with 5 % CO2. Split the cells twice per week.
- For the CD40-B cell culture: You need 1.2 x 106 cells for one 6-well plate. Resuspend the cells in wild type medium at a density of 0.1 x 106 cells/mL and irradiate them at 78 Gy. Plate 2 mL of the cell suspension into each well and incubate them at 37°C with 5 % CO2. Use this prepared plates for B-cell stimulation when tCD40L NIH/3T3 cells are adherent (at least 4 hours: check adherence with the microscope; do not wait more than 24 h to start B-cell stimulation). (Continue with B.)
B. CD40-B cell culture
I. Preparation of PBMCs for CD40-stimulation (day 0):
Please note: before you continue ascertain that feeder cells are adherent. Always add fresh solutions of interleukin-4 and cyclosporin A to the growth medium immediately before use.
- Take PBMCs, either fresh or appropriately thawed. Resuspend PBMCs twice in 50mL of 1x PBS to wash them and spin down first time at 265 x g for 7 min and a second time at 190 x g for 7 min to remove other cells. Discard supernatant and resuspend the cells in 20 mL of PBS. Determine the cell number in an aliquot of the cell suspension.
- Spin down required amount of cells at 225 x g for 5 min. For a 6-well plate 4 x 106 cells / well are needed, thus 24 x 106 cells per plate.
- Remove the supernatant and resuspend the PBMC at 1 x 106 cells/mL in CD40-B culture medium freshly supplemented with 50 U/mL of interleukin-4 as a growth factor and 0.63 μg/mL cyclosporine A to prevent outgrowth of T-cells (Given concentrations refer to one mL culture medium!).
- Remove the supernatant from 6-well plate pre-incubated with tCD40L NIH/3T3 cells.
- Wash the adherent tCD40L NIH/3T3 cells with 2 mL PBS per well first and in a second step with 2 mL of CD40-B washing medium.
- Gently add 4 mL of PBMC suspension (1 x 106 cells/mL) to each well of the 6-well plate.
- Incubate the cells at 37°C with 5 % CO2.
- On day 7, reculture the cells (Continue with 3.2.).
II. Recultivation of CD40-B cells (day 7 and then every 3-4 days):
- Harvest clusters of CD40-B cells from 6-well plate by resuspending with a 10 mL pipette and pool them into a 50 mL tube.
- Spin down at 225 x g for 7 minutes and completely replace the supernatant with CD40-B washing medium. While counting the cell amount of an aliquot, spin the cells down at 225 x g for 5 minutes. Resuspend CD40-B cells in CD40-B culture medium at a concentration of 1 x 106 cells/mL.
- Add fresh solutions of interleukin-4 in a concentration of 50 U/mL and 0.63 μg/mL cyclosporine A to the medium.
- Remove the supernatant from a 6-well plate pre-incubated with tCD40L NIH/3T3 cells.
- Wash the adherent cells with 2 mL PBS per well first and in a second step with 2 mL of CD40-B washing medium.
- Gently add 4 mL (1 x 106 cells/mL) of CD40-B suspension to each well of the 6-well plate.
- Incubate plates at 37°C with 5 % CO2.
- Subculture cells again every 3-4 days to end up with highly pure human CD40-activated B cells after a total of 14 days.
C. Trouble-shooting – What if CD40-B cells do not grow?
- Have you checked for CD40 ligand expression of feeder cells?
- The plate-bound feeder cells used for the stimulation should not be older than 24h?
- Is a contamination with mycoplasma possible?
- Was the interleukin-4 solution used for supplementation freshly thawed and had the appropriate biological activity?
- Was cyclosporin A added in the correct concentration?
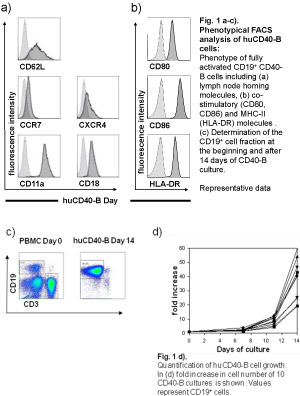
Figure 1. Please click here for a larger version of figure 1.
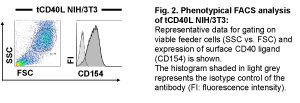
Figure 2. Please click here for a larger version of figure 2.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| A. Preparation of Media: | |||
| 1. Feeder cell wild type medium | |||
| DMEM-Ham’s / F12 | |||
| L-Glutamine (365 μg/mL) | |||
| FBS (10%) | |||
| HEPES (10 mM) | |||
| Gentamycin (15 μg/mL) | |||
| 2. Feeder cell selection medium | |||
| DMEM-Ham’s / F12 | |||
| L-Glutamine (365 μg/mL) | |||
| FBS (10%) | |||
| HEPES (10 mM) | |||
| Gentamycin (15 μg/mL) | |||
| G-418 (0.7 mg/mL) | |||
| 3. CD40-B washing medium | |||
| IMDM | |||
| L-Glutamine (584 μg/mL) | |||
| HEPES (25 mM) | |||
| Gentamycin (15 μg/mL) | |||
| 4. CD40-B culture medium | |||
| IMDM | |||
| L-Glutamine (584 μg/mL) | |||
| HEPES (25 mM) | |||
| Gentamycin (15 μg/mL) | |||
| rh Transferrin (50 μg/mL) | |||
| rh Insulin (5 μg/mL) | |||
| AB-Humanserum (10%) | |||
| B. Miscellaneous Reagents: | |||
| DMEM / Ham’s F12 | PAA Laboratories | Cat No: E15-813 | |
| IMDM | Invitrogen | REF 21980-032 | |
| Dulbecco’s PBS (10x) | PAA Laboratories | Cat No H15-011 | |
| AB-Human Serum | Invitrogen | Cat No 34005100 | |
| holo-Transferrin human | Sigma-Aldrich | Cat No T0665 | |
| Insulin human | Sigma-Aldrich | Cat No I2643 | |
| Gencin® | Delta Select | Art No 7395800 | |
| Recombinant Human Interleukin-4 | Immunotools | Cat No 11130045 | |
| Cyclosporin A | Novartis AG | Cat No NDC 0078-0109-01 | |
| FBS | Lonza Inc. | Cat No DE14-802C | |
| HEPES Buffer | PAA Laboratories | Cat No S11-001 | |
| G-418 Sulphate | PAA Laboratories | Cat No P02-012 | |
| Trypsin/EDTA (10x) | Invitrogen | REF 15400-054 | |
| C. Miscellaneous Supplies: | |||
| Sterile pipette tips | Sarstedt Ltd | ||
| 6-well plate | Nalge Nunc international | Cat No 140675 | |
| 50mL conical tube | BD Biosciences | Cat No 352070 | |
| Tissue culture flask | Sarstedt Ltd | Cat No 83.1813.002 | |
Odniesienia
- Schultze, J. L. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 100, 2757-2765 (1997).
- Schultze, J. L., Grabbe, S., vonBergwelt-Baildon, M. S. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 25, 659-664 (2004).
- Bergwelt-Baildon, M. von CD40-activated B cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants. Blood. 107, 2786-2789 (2006).
- Wiesner, M. Conditional immortalization of human B cells by CD40 ligation. PLoS ONE. 3, 1464-14 (2008).
- Urashima, M., Chauhan, D., Uchiyama, H., Freeman, G. J., Anderson, K. C. CD40 ligand triggered interleukin-6 secretion in multiple myeloma. Blood. 85, 1903-1912 (1995).
- Lapointe, R., Bellemare-Pelletier, A., Housseau, F., Thibodeau, J., Hwu, P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 63, 2836-2843 (2003).
- von Bergwelt-Baildon, M. S. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 99, 3319-3325 (2002).
- Kondo, E. CD40-activated B cells can be generated in high number and purity in cancer patients: analysis of immunogenicity and homing potential. Clin Exp Immunol. 155, 249-256 (2009).
- Mason, N. J. RNA-loaded CD40-activated B cells stimulate antigen-specific T-cell responses in dogs with spontaneous lymphoma. Gene Ther. 15, 955-965 (2008).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone