Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Zebrafish Xenograft Assay: A Method to Assess the Anti-cancer Efficacy of Therapeutics on Tumorigenicity in Zebrafish In Vivo
W tym Artykule
Overview
This video describes the zebrafish xenograft assay to assess in vivo tumor formation and study the effect of therapeutic agents.
Protokół
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Zebrafish Xenograft Assay
NOTE: This technical approach is visualized as a schematic (Figure 1).
- Preparation of stock reagents
- Prepare 1 L of 30x Danieau’s solution: 1740 mM NaCl, 21 mM KCl, 12 mM MgSO4•7H2O, 18 mM Ca(NO3)2, and 150 mM HEPES. Adjust to the pH of the buffer to 7.6 using a pH meter.
- Prepare 1% (50x) tricaine solution: Dissolve 20 mg of ethyl 3-aminobenzoate methanesulfonate (tricaine) in 2 mL of distilled water.
- Prepare 1% phenol Red-PBS solution: Add 2 µL of Phenol Red sodium salt solution to 198 µL of sterilized PBS solution in a 1.5 µL tube.
- Prepare 3% methylcellulose: Add 3 g of methylcellulose to 100 mL of 1x Danieau’s solution.
- Zebrafish egg production and incubation
- Separate one female and one male zebrafish in a partition tank filled with UV-sterilized and filtered tap water at 28.5 °C in the dark. After 24 h of separation, mix fish of both genders to initiate mating. Transfer fertilized eggs from the mating tank to a Petri dish containing 5 mL of fresh Danieau’s solution.
- Wash eggs three times with 5 mL of Danieau’s solution. For the washing, carefully aspirate the eggs with a pipette and transfer to 5 mL of fresh solution. Incubate for 8 h at 28.5 °C.
- After 8 h of incubation in Danieau's solution, change to Danieau's solution containing 0.03% 1-phenyl-2-thiourea (PTU) to inhibit pigmentation. Sort out opaque eggs (unfertilized or containing undeveloped embryos) to prevent contamination.
- Embryo dechorionation
- 24 h post fertilization (hpf), dechorionate zebrafish embryos using sharp forceps. Incubate hatching embryos in Danieau’s solution with 0.03% PTU at 28.5 °C until 48 hpf.
- Compound-treated cell preparation
- Seed A549 cells at 20,000 cells/cm2 in 25 cm2 culture flasks. After 24 h of seeding, add compounds (here OT48 at 50 µM and and/or A1210477 at 20 µM) for 24 h in an incubator at 37 °C and 5% CO2.
- Set up the treatment time for each compound to maintain the viability of the cells but to commit them towards cell death. This time point needs to be individually set up based on cell viability, morphology and molecular markers.
- 2 h before the end of the treatment, stain cells with 4 µM of fluorescent cell tracker dye CM-Dil at 37 °C and 5% CO2. After 2 h of incubation, trypsinize cells with 0.05% trypsin-EDTA, and dilute 106 cells in 50 µL of phenol Red-PBS solution prior to injection.
- Micro-injection of cancer cells for xenograft formation
- Anesthetize zebrafish in a 0.02% tricaine solution for 5 min until they settle down on an agar plate.
- Pull a 1.0 mm (outside diameter (OD) glass capillary) by a micropipette puller (Program settings: P: 300, Heat: 260, Pull: 60, VEL: 50, and Time: 200). Cut the microcapillary with a syringe to produce a sharp edge. Load microcapillaries with 20 µL of cell/phenol Red-PBS solution. Set injection pressure and time to dispense 2 nL per injection.
- Inject 100–200 cells into the yolk sac by injecting 6 to 10 nL via 3 to 5 injections. After injection, allow fish to recover for 10 to 30 min in 5 mL of fresh Danieau’s solution containing PTU solution. Dispatch fish in 24-well plates with 1 mL of Danieau's solution containing PTU solution are and incubate for 72 h at 28.5 °C.
- After 72 h of incubation, anesthetize fish in a 0.02% tricaine solution and immobilize on a glass slide with a drop of 3% methylcellulose. Take 5x pictures by fluorescence microscopy at a wavelength of 620 to 750 nm.
- Quantify the size of fluorescent tumors by ImageJ software. Select menu "Analyze | Measure". Save values corresponding to the fluorescent signal and analyze with a statistics software for graphical representation.
Wyniki
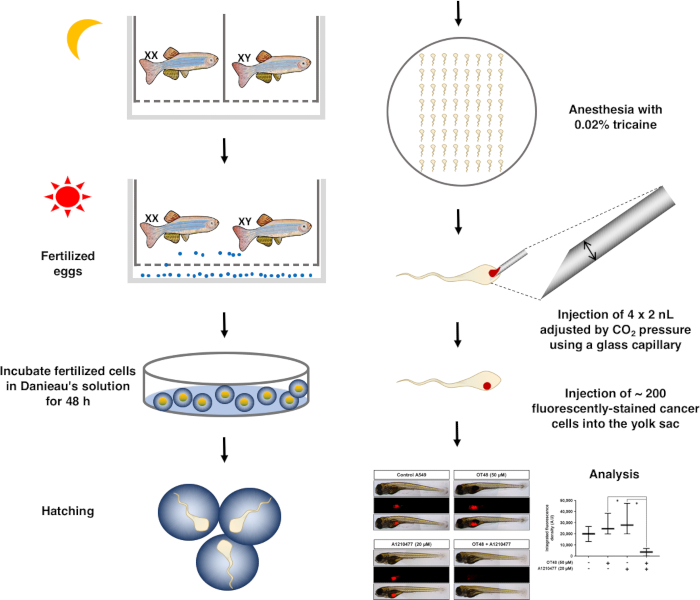
Figure 1: Schematic diagram of the overall process of the zebrafish xenograft assay.
Ujawnienia
Materiały
| Name | Company | Catalog Number | Comments |
| A549 | ATCC | CCL-185 | 37 C° |
| RPMI 1640 | Lonza | 30096 | 4 C° |
| FBS | Biowest | S1520-500 | -20 C° |
| Penicillin-streptomycin | Lonza | 17-602E | -20 C° |
| Cell culture flask T25 | SPL | 70025 | RT |
| Cell culture flask T75 | SPL | 70075 | RT |
| Cell culture flask T175 | SPL | 71175 | RT |
| 1.5ml tube | Extragene | Tube-170-C | RT |
| 24 well plate | SPL | 30024 | RT |
| Petridish | SPL | 10100 | RT |
| PBS solution | Hyclone | SH30256.02 | RT |
| Trypsin-EDTA | Gibco | 25-300-054 | 4 C° |
| Sodium Chloride | Sigma-Aldrich | 71382 | RT |
| Potassium chloride (KCL) | Sigma-Aldrich | P9541 | RT |
| Magnesium sulfate heptahydrate (MgSO4.7H2O) | Sigma-Aldrich | M2773 | RT |
| Calcium nitrate tetrahydrate (Ca(NO3)2) | Sigma-Aldrich | C1396 | RT |
| HEPES solution | Sigma-Aldrich | H0887 | RT |
| Ethyl 3-aminobenzoate methanesulfonate (Tricaine) | Sigma-Aldrich | E10521 | RT |
| Phenol Red solution | Sigma-Aldrich | P0290 | RT |
| Methylcellulose | Sigma-Aldrich | M0512 | RT |
| 1-phenyl-2-thiourea (PTU) | Sigma-Aldrich | P7629 | RT |
| CM-Dil dye | Invitrogen | C7001 | -20 C° |
| Glass capillary | World Precision Instruments | TW 100F-4 | RT |
| Micropipette puller | Shutter instrument, USA | P-97 | RT |
| Micro injector | World Precision Instruments | PV820 | RT |
| Syringe | KOVAX | 1ml | RT |
| Micro loader | Eppendorf | 5242956003 | RT |
| Glass slide | Marienfeld | HSU-1000612 | RT |
| Fluorescence microscopy | Leica | DE/DM 5000B | RT |
This article has been published
Video Coming Soon
Source: Lee, J. Y et al.,Preclinical Assessment of the Bioactivity of the Anticancer Coumarin OT48 by Spheroids, Colony Formation Assays, and Zebrafish Xenografts. J. Vis. Exp. (2018)
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone