Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Studying the Effects of Matrix Stiffness on Cellular Function using Acrylamide-based Hydrogels
W tym Artykule
Podsumowanie
The effect of substrata stiffness on cellular function can be modeled in vitro using polyacrylamide hydrogels of varying compliances.
Streszczenie
Tissue stiffness is an important determinant of cellular function, and changes in tissue stiffness are commonly associated with fibrosis, cancer and cardiovascular disease1-11. Traditional cell biological approaches to studying cellular function involve culturing cells on a rigid substratum (plastic dishes or glass coverslips) which cannot account for the effect of an elastic ECM or the variations in ECM stiffness between tissues. To model in vivo tissue compliance conditions in vitro, we and others use ECM-coated hydrogels. In our laboratory, the hydrogels are based on polyacrylamide which can mimic the range of tissue compliances seen biologically12. "Reactive" cover slips are generated by incubation with NaOH followed by addition of 3-APTMS. Glutaraldehyde is used to cross-link the 3-APTMS and the polyacrylamide gel. A solution of acrylamide (AC), bis-acrylamide (Bis-AC) and ammonium persulfate is used for the polymerization of the hydrogel. N-hydroxysuccinimide (NHS) is incorporated into the AC solution to crosslink ECM protein to the hydrogel. Following polymerization of the hydrogel, the gel surface is coated with an ECM protein of choice such as fibronectin, vitronectin, collagen, etc.
The stiffness of a hydrogel can be determined by rheology or atomic force microscopy (AFM) and adjusted by varying the percentage of AC and/or bis-AC in the solution12. In this manner, substratum stiffness can be matched to the stiffness of biological tissues which can also be quantified using rheology or AFM. Cells can then be seeded on these hydrogels and cultured based upon the experimental conditions required. Imaging of the cells and their recovery for molecular analysis is straightforward. For this article, we define soft substrata as those having elastic moduli (E) <3000 Pascal and stiff substrata/tissues as those with E >20,000 Pascal.
Protokół
Preparation
- Coverslips should be autoclaved.
- Sterile distilled or deionized water should be used to prepare solutions and for washing coverslips.
- AC (40% w/v) and bis-AC (1% w/v) solutions are sterilized by 0.2 μm filtration. Prepare 10% ammonium persulfate (APS; 100μg/ml water) shortly before use and sterile filter. Replace the APS solution monthly.
- Chemical reagents such as 3-APTMS, chloroform, glutaradehyde, NHS, and SurfaSil that cannot be autoclaved are kept in an assigned bottle used solely for the preparation of hydrogels.
- For best results, hydrogels should be utilized within a couple of days after the overnight incubation with the appropriate ECM protein.
- Prepare a 10% SurfaSil solution in chloroform (10 ml is usually enough for 20 top coverslips) in a 50 ml polypropylene Falcon tube prior to pouring of hydrogel. (Our lab usually siliconizes the top coverslips during the 0.5% glutaraldehyde incubation step). Add the coverslips to the Falcon tube and rock for at least 10 min. Decant the SurfaSil solution and air dry the coverslips on Kimwipes in the biological safety cabinet where the hydrogels will be prepared.
- Prepare the heat-inactivated BSA solution as follows: a 20 mg/ml solution of fatty-acid free BSA in PBS is incubated in a 68°C water bath for 30 min. The solution is then sterile filtered and stored at 4°C.
Procedure
- Place a layer of Parafilm on the bottom half of a 150-mm Petri dish.
- Place up to 9 25-mm coverslips on top of the Parafilm and cover them with 1 ml 0.1 M NaOH. Incubate for 3 min and then aspirate with a vacuum line.
- Working in a chemical hood, place 0.5 ml 3-APTMS on each coverslip. Incubate for 3 min and then aspirate the APTMS. If you wait too long, a foam will form.
- Rinse the coverslips once with 20 ml deionized water in the same dish. Remove the coverslips from the dish using curved forceps and transfer them, with their treated-side facing up, to a new 150-mm dish. Wash the coverslips with deionized water three times, on the rocker, for 10 min each wash. If you fail to remove all the APTMS, it will react with the glutaraldehyde in the next step and leave a white cloudy precipitate (Figure 1).
- Thaw the glutaraldehyde (~10 min before use).
- Using curved forceps, transfer the coverslips to a clean dish layered with Parafilm and aspirate any remaining liquid. Use a vacuum line or Kimwipe to blot the remaining water as needed.
- Cover each coverslip completely with 0.5 ml of 0.5% glutaraldehyde in sterile deionized water and incubate for 30 min in a chemical hood. Aspirate the glutaraldehyde. Rinse and wash the coverslips as in step 4. Dry the coverslips completely. [You can stop here and leave the coverslips for several weeks in a dry area].
- When you are ready to prepare the hydrogels, use curved forceps to transfer the coverslips, reactive side up, to a sheet of Parafilm that has been taped onto the surface of the biological safety cabinet. Make sure that the coverslips are flat on the Parafilm surface.
- Prepare a saturated NHS solution in toluene. (Dissolve a small amount of NHS in enough toluene for the particular experiment. Keep adding NHS bit by bit until the NHS no longer dissolves. The saturated solution is usually cloudy and pink.)
- Next, prepare the acrylamide, bis-acrylamide, water and APS to reach the desired acrylamide percentage. Add the reagents in microcentrifuge tubes as outlined below to a total volume of 0.8ml.
- Then, one aliquot at a time, add the NHS and TEMED to the 0.8 ml AC solution, vortex briefly and IMMEDIATELY pour 3-5 gels using 140 μl per coverslip in the biological safety hood. Whenever possible, all steps from this point should be performed in a biological safety cabinet. [Note: different size coverslips can be utilized based upon need. If 18-mm coverslips are used, use ~33 μl AC solution per coverslip and an 18-mm top siliconized coverslip.]
Bis-AC (%) 0.3 (stiff) 0.15 0.06 0.03 (soft) μl μl μl μl Water 402 522 594 618 AC 150 150 150 150 Bis-AC 240 120 48 24 APS 8 8 8 8 TEMED 1 1 1 1 NHS 228 228 228 228 - Quickly place the siliconized 25-mm coverslip on top of each gel before it begins to polymerize. Addition of the top coverslip should allow the AC to completely cover the bottom coverslip. Incubate this "sandwich" at room temperature until the AC polymerizes. (Check the residual AC solution in the microcentrifuge tube to determine when polymerization has occurred. Usually a few minutes for stiff gels and a little longer for soft ones will suffice.).
- Carefully pick up the sandwich (sterile gloves on!) and slide off the top coverslip until it overhangs the polymerized gel. You can then pry it off the gel. If you wait too long before removing the top coverslip, the gel will rip as the coverslip is removed.
- Discard the top coverslip. Place the bottom gel-coverslips (hereafter called the hydrogel) into 6-well plates with 2 ml PBS/well. PBS can be added before or after the gel-coverslip to each well. Wash the hydrogels with PBS three times, on a rocker, 5 min per wash.
- Repeat steps 11-14 until the required number of hydrogels have been prepared.
- Cover each hydrogel with 2 ml of a fibronectin solution (3 μg/ml in PBS) or other ECM protein (see Klein et al.13 for details.). The ECM protein becomes covalently bound to the hydrogel during overnight incubation at 4°C.
- Aspirate the ECM solution and block unreacted NHS with 1mg/ml heat-inactivated fatty-acid free BSA in serum-free media for at least 30 min at 37°C in a cell culture incubator. Rinse the hydrogels once with sterile PBS or cell culture medium.
- Plate cells in the appropriate culture medium containing FBS. The number of cells seeded on the hydrogel should be determined by the user based upon the degree of cell spreading and confluency required for experimentation. Approximately 105 cells is usually sufficient for western blotting and qPCR analysis.
- Following the incubation period, cell protein or mRNA can be extracted. The coverslips are carefully removed from the wells using curved forceps and placed (cell-side down) on top of 100 μl droplets of lysis buffer which have been spaced out (2-3cm) along a sheet of Parafilm on the lab bench. (We use standard SDS sample buffer and TRIzol, respectively, to prepare samples for western blotting and to isolate RNA for qPCR.) Incubate the cells with the lysis buffer for exactly one minute. Remove the coverslips and transfer the lysis buffer to a microcentrifuge tube. Alternatively for RNA extraction only, the user can transfer each hydrogel to a new 6-well plate and add 1ml of TRIzol/well. Incubate for 3 minutes and remove TRIzol solution for storage in a microcentrifuge tube.
Additional information on procedures such as immunofluorescence, BrdU staining, transfection, etc. for cells seeded on hydrogels is described in Klein et al. 200713.
Representative Results
Thorough washing of the coverslips following addition of APTMS is an important step in producing "reactive" coverslips. If one fails to remove the APTMS completely, it will react with the glutaraldehyde in the following step and produce a white cloudy precipitate as seen in Figure 1A. Figure 1B shows a properly washed and dried coverslip. If the precipitate develops, the whole procedure must be restarted from the beginning as the coverslip is no longer useable.
Following hydrogel formation and coating with ECM proteins overnight, cells can be seeded the following day. As Figure 2 shows, there is a distinct difference between cell spreading on stiff versus soft hydrogels. As can be seen by phalloidin staining in mouse embryonic fibroblasts (MEFs), cells spread to a greater extent on stiff as compared to soft hydrogels. Indeed, most cells attaching to a soft hydrogel will remain compact and attach less efficiently.
Although only MEF morphology is shown in Figure 2, the difference in cell spreading is consistent across several other cell lines tested11-12,14.
Secrets to Success
- Once the procedure is started, it is very important to keep the coverslips with the "reactive" side up and keep in mind which side has been coated.
- Gloves should be worn at all time during the procedure to provide a working environment that is as sterile as possible.
- Most of the initial steps are performed between a chemical fume hood and the lab bench. All steps subsequent to removing excess glutaraldehyde from the coverslips should be executed under a biological safety cabinet.
- Due to differences in cell spreading (Figure 2), we recommend seeding twice the number of cells on soft hydrogels as compared to stiff hydrogels.
- The AC polymerization process is extremely quick. For first time users, one can decrease the amount of APS or TEMED in the solution to prolong the polymerization process. Do not try to prepare more than a few coverslips at one time.
- In cell cycle studies where synchronization in G0 is required, we usually serum-starve cells in plastic culture dishes, trypsinize the cells and reseed them on hydrogels of different compliances in the presence of mitogens (FBS and/or growth factors). This procedure ensures that the starting population of G0-synchronized cells is identical in all samples. However, for many signal transduction studies, the required mitogen stimulation period may not be long enough to allow for attachment and spreading of cells. In this case, we serum-starve the cells on the hydrogels and then directly stimulate them with mitogen.

Figure 1A. Improperly washed coverslip. Following addition of APTMS, coverslip was washed for 1-2 minutes before addition of the glutaraldehyde solution. A precipitate forms on the coverslip that has not been washed according to the steps outlined in Procedure.

Figure 1B. Properly washed coverslip. Following addition of APTMS, coverslip was washed for three times 10 minutes each before addition of the glutaraldehyde solution. No precipitate forms on the coverslip.
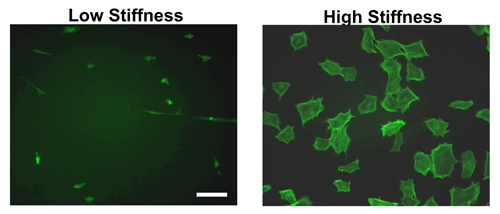
Figure 2. Morphology of cells on hydrogels of varying stiffness. Established MEFs were seeded on fibronectin-coated hydrogels of high or low stiffness for 9 hours. Following the incubation period, the cells were fixed, permeabilized and stained with FITC-phalloidin which binds to f-actin. MEFs on high stiffness gels exhibit stress fibers and are well spread compared to those seeded on low stiffness hydrogels. Scale bar= 50μm.
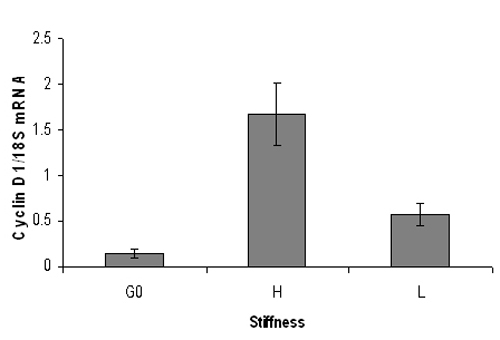
Figure 3. Representative quantitative PCR result of mouse cyclin D1 mRNA levels. Serum starved mouse embryonic fibroblasts were plated onto high (3% acrylamide) or low (0.3% acrylamide) stiffness hydrogels and stimulated with 10% FBS for 9 hrs. Following RNA extraction, real-time quantitative PCR analysis was performed for cyclin D1 mRNA levels (normalized to 18S RNA). G0 represents cyclin D1 mRNA from quiescent cells. Cyclin D1 mRNA levels increase significantly on stiff hydrogels but not on soft hydrogels. Data are mean +/- SD of duplicate PCR reactions.
Dyskusje
A crucial element of the hydrogel polymerization process is to avoid air bubble formation which will allow cells to bind to the glass coverslip rather than the ECM-coated hydrogel itself. This can be prevented by carefully pipetting the polymerization solution after vortexing and visually making sure that no air bubbles have become trapped in the gel. We always recommend preparing additional "reactive" coverslips and hydrogels to ensure having enough for experimentation.
Particular attention ...
Ujawnienia
No conflicts of interest declared.
Podziękowania
Work is our laboratory is supported by grants from the National Institutes of Health.
Materiały
| Name | Company | Catalog Number | Comments |
| Glutaraldehyde, 70% | Sigma-Aldrich | G7776 | Store at -20°C |
| 3-APTMS (3-Aminopropyltrimethosysilane 97%) | Sigma-Aldrich | 281778 | Store at room temperature |
| SurfaSil Siliconizing Fluid | Thermo Fisher Scientific, Inc. | 42800 | Store at room temperature |
| NHS (N-hydroxysucinimide Ester) | Sigma-Aldrich | A-8060 | Store at 4°C Replace monthly |
| Albumin, bovine serum, essentially fatty acid free | Sigma-Aldrich | A6003-100G | Store at 4°C |
| Coverslips (25mm) | Fisher Scientific | 12-545-86 25 Cir 1D | |
| Coverslips (18mm) | Fisher Scientific | 12-545-84 18 Cir 1D |
Odniesienia
- Beattie, D., Xu, C., Vito, R., Glagov, S., Whang, M. C. Mechanical analysis of heterogeneous, atherosclerotic human aorta. J Biomech Eng. 120, 602-607 (1998).
- Bernini, G. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 26, 2399-2405 (2008).
- Boonyasirinant, T. Aortic stiffness is increased in hypertrophic cardiomyopathy with myocardial fibrosis: novel insights in vascular function from magnetic resonance imaging. J Am Coll Cardiol. 54, 255-2562 (2009).
- Discher, D. E., Janmey, P., Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science. 310, 1139-1143 (2005).
- Duprez, D. A., Cohn, J. N. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep. 9, 139-144 (2007).
- Lee, R. T. Prediction of mechanical properties of human atherosclerotic tissue by high-frequency intravascular ultrasound imaging. An in vitro study. Arterioscler Thromb. 12, 1-5 (1992).
- Levental, K. R. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 139, 891-906 (2009).
- Paszek, M. J. Tensional homeostasis and the malignant phenotype. Cancer Cell. 8, 241-254 (2005).
- Samani, A., Zubovits, J., Plewes, D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol. 52, 1565-1576 (2007).
- Wells, R. G. The role of matrix stiffness in regulating cell behavior. Hepatology. 47, 1394-1400 (2008).
- Pelham, R. J., Wang, Y. -. L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad Sci USA. 94, 13661-13665 (1997).
- Klein, E. A., Yung, Y., Castagnino, P., Kothapalli, D., Assoian, R. K. Cell adhesion, cellular tension, and cell cycle control. Methods Enzymol. 426, 155-175 (2007).
- Klein, E. A. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Current Biology. 19, 1511-1518 (2009).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone