Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
In vivo and in vitro Studies of Adaptor-clathrin Interaction
W tym Artykule
Podsumowanie
Clathrin-mediated endocytosis depends on adaptor proteins that coordinate cargo selection and clathrin coat assembly. Here we describe procedures to study adaptor-clathrin physical interaction and live cell imaging approaches using as a model the yeast endocytic adaptor protein Sla1p.
Streszczenie
A major endocytic pathway initiates with the formation of clathrin-coated vesicles (CCVs) that transport cargo from the cell surface to endosomes1-6. CCVs are distinguished by a polyhedral lattice of clathrin that coats the vesicle membrane and serves as a mechanical scaffold. Clathrin coats are assembled during vesicle formation from individual clathrin triskelia , the soluble form of clathrin composed of three heavy and three light chain subunits7,8. Because the triskelion does not have the ability to bind to the membrane directly, clathrin-binding adaptors are critical to link the forming clathrin lattice to the membrane through association with lipids and/or membrane proteins9. Adaptors also package transmembrane protein cargo, such as receptors, and can interact with each other and with other components of the CCV formation machinery9.
Over twenty clathrin adaptors have been described, several are involved in clathrin mediated endocytosis and others localize to the trans Golgi network or endosomes9. With the exception of HIP1R (yeast Sla2p), all known clathrin adaptors bind to the N-terminal -propeller domain of the clathrin heavy chain9. Clathrin adaptors are modular proteins consisting of folded domains connected by unstructured flexible linkers. Within these linker regions, short binding motifs mediate interactions with the clathrin N-terminal domain or other components of the vesicle formation machinery9. Two distinct clathrin-binding motifs have been defined: the clathrin-box and the W-box9. The consensus clathrin-box sequence was originally defined as L[L/I][D/E/N][L/F][D/E]10 but variants have been subsequently discovered11. The W-box conforms to the sequence PWxxW (where x is any residue).
Sla1p (Synthetic Lethal with Actin binding protein-1) was originally identified as an actin associated protein and is necessary for normal actin cytoskeleton structure and dynamics at endocytic sites in yeast cells12. Sla1p also binds the NPFxD endocytic sorting signal and is critical for endocytosis of cargo bearing the NPFxD signal13,14. More recently, Sla1p was demonstrated to bind clathrin through a motif similar to the clathrin box, LLDLQ, termed a variant clathrin-box (vCB), and to function as an endocytic clathrin adaptor15. In addition, Sla1p has become a widely used marker for the endocytic coat in live cell fluorescence microscopy studies16. Here we use Sla1p as a model to describe approaches for adaptor-clathrin interaction studies. We focus on live cell fluorescence microscopy, GST-pull down, and co-immunoprecipitation methods.
Protokół
1. Incorporation of a GFP tag and selectable marker in the SLA1 gene
Apply the Longtine method17 in order to fuse a GFP tag directly at the 3' end of the SLA1 gene open reading frame (Sla1p C-terminus) and simultaneously mark the gene with the E. coli kanr gene that allows for G418 selection.
- Generate a DNA fragment by PCR using as a template the plasmid pFA6a-GFP(S65T)-kanMX618 and the following primers: forward, 5'-CA AGG CAA GCC AAC ATA TTC AAT GCT ACT GCA TCA AAT CCG TTT GGA TTC CGG ATC CCC GGG TTA ATT AA-3', and reverse, 5'-CA TAT AGC TTG TTT TAG TTA TTA TCC TAT AAA ATC TTA AAA TAC ATT AAT GAA TTC GAG CTC GTT TAA AC-3'. The underlined sequence corresponds to the SLA1 gene specific segment immediately preceding (forward primer) and following the stop codon (reverse primer). Isolate the PCR product by agarose gel electrophoresis followed by DNA purification.
- Prepare a 50 ml culture S. cerevisiae SEY6210 strain (MAΤα ura3-52, leu2-3,112 his3-Δ200, trp1-Δ901, lys2-801, suc2-Δ9 GAL-MEL) 19 in YPD, growing at early logarithmic phase (OD600 =0.2-0.6). Spin at room temperature for 3 min at 2,000 x g, wash twice with sterile water.
- Transform the PCR fragment obtained in step 1 into the cells using the lithium acetate procedure20. Wash the cells with 1 ml sterile water, add 1 ml YPD and incubate for 4 h at 30 °C in a shaker.
- Spread the cells on YPD-G418 plates to select for G418-resistant transformants, incubate at 30 °C for 2-3 days. Pick colonies and streak them on YPD-G418 plates.
- Using colony-PCR, identify transformants in which the GFP-kan module was properly integrated with the SLA1 gene sequences by homologous recombination. Use a forward primer that anneals within the SLA1 open reading frame and a reverse primer that anneals inside the GFP-kan module. Identify colonies which PCR products have the expected size and verify by sequencing.
- Screen the colonies by fluorescence microscopy to confirm they have GFP fluorescence.
2. Mutation of the variant clathrin box (vCB) in the SLA1 gene
- Introduce an LLDLQ to AAALQ mutation in the SLA1 gene (nucleotides 2407-2415) following a two step approach15.
- First, amplify SLA1 nucleotides 1207-1410 and 2427-2589 by PCR and clone the fragments into NotI/BamHI and EcoRI/SalI sites of pBluescriptKS, respectively.
- Subclone into the BamHI/EcoRI sites a PCR fragment containing URA3.
- Cleave the resulting plasmid with NotI/SalI, isolate the URA3 fragment by gel purification, and introduce it by lithium acetate transformation into the Sla1-GFP strain generated in Part 1, or into TVY614 (MATa ura3-52 leu2-3112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 pep4::LEU2 prb1::HISG prc1::HIS3)21.
- Spread the cells on supplemented SD plates lacking uracil. Confirm by colony-PCR and sequencing that the Ura+ colonies contain properly integrated URA3 replacing the vCB fragment.
- In a second step, subclone SLA1 nucleotides 1207-2589 into NotI/SalI sites of pBluescriptKS. Using the QuickChange-XL site-directed mutagenesis kit (Stratagene), mutate the vCB residues LLDLQ to AAALQ. Verify by sequencing.
- Cleave the resulting construct with BsgI/AgeI, and isolate the mutant vCB containing fragment by gel purification. Cotransform the BsgI/AgeI fragment with pRS313 (HIS3)22 into the strain obtained in Part 2.3.
- Spread the cells on supplemented SD plates lacking histidine. Replica-plate His+ colonies onto agar medium containing 5-fluorotic acid to identify cells in which the mutant sequences replaced URA3, thus regenerating the sla1 gene containing the LLDLQ to AAALQ mutation (sla1AAA). Confirm by colony-PCR and sequencing.
3. Fluorescence microscopy
- Grow the yeast strains Sla1-GFP and sla1AAA-GFP generated in Parts 1 and 2 in 4 ml of supplemented SD media at 30°C in a rotator in the dark until reaching an OD600 = 0.1-0.4. Spin 1ml of culture in microcentrifuge at 4,000 x g for 1 min at room temperature. Discard ~950 μl of supernatant. Resuspend cells in the remaining liquid (~50 μl).
- Deposit 3 μl of the cell suspension onto a microscopy slide and cover with a glass coverslip. Protect the sample from light.
- Mount slide on a 100x oil-immersion objective of a spinning-disc confocal microscope.
- Locate correct focal plane of cells using brightfield illumination or 488 nm laser with appropriate filters to excite and detect fluorescence from GFP.
- Capture time-lapse images of Sla1-GFP and sla1AAA-GFP in cells with exposure time of 500 ms (or appropriate value) at an interval of one image per second for 150 seconds. Expected result: GFP labeled punctae will appear on the inner surface of limiting membrane of the cell, persist for several seconds, and move toward the center of the cell as the signal quickly disappears (indicating coat disassembly).
- Select a portion of an image in which spots are seen to appear, remain for several seconds, and disappear. Crop this section of the image for each frame of the time-lapse images.
- Generate a kymograph representing this section of the image in each frame of the 150 second time-lapse image by aligning the frames along an axis corresponding to time elapsed.
- Calculate the duration of each spot based on how many seconds (time-lapse frames) the spots is present in the kymograph. Note that each spot should "curve" toward the interior of the cell as it is disappearing, reflecting endocytosis and disassembly of the coat.
- Compare wild type Sla1-GFP with sla1AAA-GFP. Repeat to determine if results are statistically significant. Expected result: sla1AAA-GFP spots persist significantly longer (~80 sec) than wild type (~30 sec) indicating a defect in coat formation15.
4. Preparation of yeast cytosolic extract and total cell extract
- Inoculate 3 liters of YPD with an appropriate yeast strain, such as TVY614 21, and grow at 30 °C in a shaker incubator until reaching an OD600 = 1-2.
- Centrifuge the yeast culture at room temperature for 20 min at 3,700 x g. Discard the supernatant, add 3-5 ml of sterile water, and pipette up and down until the pellet is completely resuspended.
- Pour ~150 ml of liquid nitrogen in a 250 ml plastic beaker. Flash-freeze the yeast by adding dropwise into the liquid nitrogen in a circular pattern to avoid agglutination of the frozen pellets. Do not let the pellets thaw, add more liquid nitrogen if necessary.
- Chill a stainless steel blender container by pouring liquid nitrogen in it. Allow the liquid nitrogen to almost completely evaporate. Add the frozen yeast pellets to the cold blender container. Close the blender container with a cold rubber stopper and grind the yeast pellets for 10 seconds. Invert the container 3-4 times to mix contents and repeat the grinding step twice. The ground frozen yeast will have a powder-like appearance.
- Chill a funnel and a 50 ml conical tube with liquid nitrogen. Using the cold funnel, transfer the ground yeast to the tube. The frozen ground yeast can be store at -80 °C or used immediately.
- To obtain a cytosolic extract for the GST-fusion protein affinity assay (Part 5), weight 3 g of frozen ground TVY614 yeast in a 15 ml conical tube and resuspend in 3 ml of room temperature buffer A (10 mM HEPES, pH 7.0, 150 mM NaCl, 1 mM EDTA, 1 mM DTT) containing protease inhibitor cocktail (Sigma).
- Cap and invert the tube several times until completely thawed and then put on ice.
- Ultracentrifuge the extract at 4 °C for 20 min at 300,000 x g. Carefully transfer the supernatant (cytosolic extract) to a conical tube using a pipette without disturbing the pellet. Keep the cytosolic extract on ice.
- Add 100 μl of a 50% (v/v) glutathione-Sepharose slurry in buffer A. It is convenient to cut the end of the tip in order to facilitate pipeting the beads. Rotate at 4 °C for 15 min, spin-down the beads by centrifugation at 4 °C for 2 min at 1,000 x g, and transfer the supernatant (cytosolic extract) to a fresh tube. Reserve 50 μl of extract for input control.
- To obtain total cell extracts for co-immunoprecipitation experiments (Part 6), use strain TVY614 (WT SLA1), the sla1AAA strain carrying an LLDLQ to AAALQ mutation generated in Part 2 in TVY614 background, and a sla1 strain carrying a deletion of the SLA1 gene such as GPY3130 23. Weight 2 g of the corresponding frozen ground yeast in conical tubes and resuspend them in 2 ml of room temperature buffer A, containing protease inhibitor cocktail (Sigma) and 2% Triton X-100.
- Cap and invert the tube several times until completely thawed and then incubate on ice for 10 min, periodically mixing by inversion.
- Transfer the material to microcentrifuge tubes and spin at 4 °C for 15 min at 16,000 x g (top speed in microcentrifuge). Carefully transfer the supernatant (total cell extract) to a conical tube on ice using a pipette without disturbing the pellet.
- Add 100 μl of a 50% (v/v) protein A-Sepharose slurry in buffer A. Rotate at 4 °C for 15 min, spin-down the beads by centrifugation at 4 °C for 2 min at 1,000 x g, and transfer the supernatant (total extract) to a fresh tube. Reserve 50 μl of extract for input control.
5. GST-fusion protein affinity assay
- Amplify by PCR a fragment containing the Sla1p variant clathrin-box (vCB) (residues 803-807), clone it into pGEX-5X to obtain pGEX-5X-vCB. Verify by sequencing.
- Using pGEX-5X-vCB as a template and the QuickChange-XL site-directed mutagenesis kit (Stratagene), mutate the vCB residues LLDLQ to AAALQ to obtain pGEX-5X-vCBmut. Verify by sequencing.
- Express GST and the fusion proteins GST-vCB and GST-vCBmut in E. coli (BL21 DE3), purify using glutathione-Sepharose, elute from the beads with reduced glutathione, dialyze against PBS, and determine protein concentration.
- Label 3 microcentrifuge tubes: GST, GST-vCB, and GST-vCBmut, and add 1 ml of PBS, 30 μl of a 50% (v/v) glutathione-Sepharose slurry in buffer A, and 50 μg of dialized GST, GST-vCB, or GST-vCBmut fusion proteins.
- Rotate at room temperature for 30 min to allow for binding.
- Wash the beads 2 times with PBS and 1 time with buffer A. Add 1 ml of yeast cytosolic extract - prepared as described in Part 4 - to each tube.
- Rotate the tubes 1 h at 4 °C, spin 10 sec at 5,000 x g to pellet the beads, discard supernatant, and quickly wash the beads 3 times with buffer A containing 0.1% Triton X-100, and 1 time with buffer A.
- Spin down the beads one more time and using a gel loading tip remove as much liquid as possible. At this point the samples can be stored at -20 °C to continue at a later time.
- Add 15 μl of 2x Laemmli sample buffer to each tube, incubate at 96 °C for 5 min, and spin 10 sec at 5,000 x g.
- Analyze the samples by immunoblotting using an antibody to the clathrin heavy chain. Expected results: clathrin binds to GST-vCB but not to GST or GST-vCBmut. Also analyze the samples by SDS-PAGE to confirm similar loading of the GST fusion proteins.
6. Co-immunoprecipitation assay
- Label 3 microcentrifuge tubes: WT (wild type SLA1), sla1AAA, and sla1Δ, and add 1 ml of PBS, 30 μl of a 50% (v/v) protein A-Sepharose slurry in buffer A, and 2 μg of a rabbit anti-Sla1p antibody.
- Rotate at room temperature for 30 min.
- Wash the beads twice with PBS and 1 time with buffer A containing 1% Triton X-100.
- Add 1 ml of the corresponding total yeast extracts prepared in Part 4: SLA1, sla1AAA, and sla1Δ. Rotate 1 h at 4 °C.
- Spin 10 sec at 5,000 x g to pellet the beads, discard supernatant, and quickly wash the beads 3 times with buffer A containing 0.1% Triton X-100, and 1 time with buffer A.
- Spin down the beads one more time and using a gel loading tip remove as much liquid as possible. At this point the samples can be stored at -20 °C to continue at a later time.
- Add 15 μl of 2x Laemmli sample buffer to each tube, incubate at 96 °C for 5 min, and spin 10 sec at 5,000 x g.
- Analyze the samples by immunoblotting using an antibody to the clathrin heavy chain. Expected results: clathrin co-immunoprecipitates with wild type Sla1p but not to with sla1AAA, or in the sla1Δ sample.
7. Representative Results
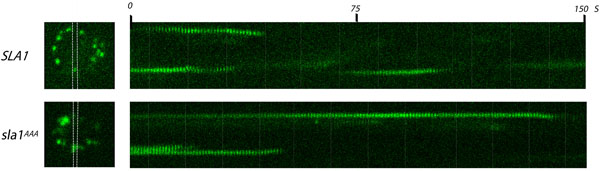
Figure 1. Analysis of Sla1p clathrin adaptor at endocytic sites by live cell fluorescence microscopy. One frame (left) from a video and corresponding kymographs (right) of yeast cells expressing Sla1-GFP or sla1AAA-GFP carrying an LLDLQ to AAALQ mutation. Both wild type and mutant Sla1-GFP were expressed from the endogenous SLA1 locus. Endocytic sites are observed as bright spots distributed in the cell periphery (left images). The area between white lines corresponds to the region from which kymographs were generated. Movies were taken at a frame rate of 1 frame/sec. Notice the longer lifetime of Sla1-GFP in the LLDLQ to AAALQ mutant (sla1AAA) compared to wild type (SLA1).
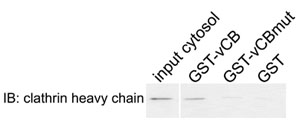
Figure 2. Physical interaction between clathrin and the Sla1p-variant clathrin box. GST fused to Sla1p fragment aa798-813 containing the sequence LLDLQ (GST-vCB), the corresponding AAALQ mutant (GST-vCBmut), or GST alone (GST) were bound to glutathione-Sepharose beads and incubated with a cytosolic extract from wild-type yeast cells. The associated proteins were eluted and analyzed by immunoblotting (IB) for clathrin heavy chain.
Dyskusje
Clathrin-coated vesicles (CCV) participate in endocytosis and transport from the trans Golgi network and endosomes, conserved pathways that are fundamental to eukaryotic cell biology. The approaches described in this methods paper are useful to study the molecular mechanisms responsible for CCV formation, in particular interactions between adaptor proteins and clathrin. GST-fusion protein affinity and co-immunoprecipitation assays allow testing for physical association between an adaptor (candidate) and clathrin...
Ujawnienia
No conflicts of interest declared.
Podziękowania
D.F. is supported by an NSF Bridges to the Doctorate fellowship. The microscope used in this work is supported in part by the Microscope Imaging Network core infrastructure grant from Colorado State University. Work on clathrin adaptors in the author s laboratory is supported by CSU start-up funds and American Heart Association award 09SDG2280525 to S.D.
Materiały
| Name | Company | Catalog Number | Comments | |
| QuickChange-XL site-directed mutagenesis kit | Stratagene | |||
| protease inhibitor cocktail | Sigma |
Odniesienia
- Mellman, I., Warren, G. The road taken: past and future foundations of membrane traffic. Cell. 100, 99-112 (2000).
- Engqvist-Goldstein, A. E., Drubin, D. G. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol. 19, 287-332 (2003).
- Conner, S. D., Schmid, S. L. Regulated portals of entry into the cell. Nature. 422, 37-44 (2003).
- Bonifacino, J. S., Traub, L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 72, 395-447 (2003).
- Ungewickell, E. J., Hinrichsen, L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 19, 417-425 (2007).
- Doherty, G. J., McMahon, H. T. Mechanisms of endocytosis. Annu Rev Biochem. 78, 857-902 (2009).
- Kirchhausen, T. Clathrin. Annu Rev Biochem. 69, 699-727 (2000).
- Brodsky, F. M., Chen, C. Y., Knuehl, C., Towler, M. C., Wakeham, D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 17, 517-568 (2001).
- Owen, D. J., Collins, B. M., Evans, P. R. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 20, 153-191 (2004).
- Dell'Angelica, E. C., Klumperman, J., Stoorvogel, W., Bonifacino, J. S. Association of the AP-3 adaptor complex with clathrin. Science. 280, 431-434 (1998).
- Dell'Angelica, E. C. Clathrin-binding proteins: got a motif? Join the network!. Trends Cell Biol. 11, 315-318 (2001).
- Holtzman, D. A., Yang, S., Drubin, D. G. Synthetic-lethal interactions identify two novel genes, SLA1 and SLA2, that control membrane cytoskeleton assembly in Saccharomyces cerevisiae. J Cell Biol. 122, 635-644 (1993).
- Howard, J. P., Hutton, J. L., Olson, J. M., Payne, G. S. Sla1p serves as the targeting signal recognition factor for NPFX(1,2)D-mediated endocytosis. J. Cell. Biol. 157, 315-326 (2002).
- Mahadev, R. K., Pietro, S. M. D. i., Olson, J. M., Piao, H. L., Payne, G. S., Overduin, M. Structure of Sla1p homology domain 1 and interaction with the NPFxD endocytic internalization motif. EMBO J. 26, 1963-1971 (2007).
- Pietro, S. M. D. i., Cascio, D., Feliciano, D., Bowie, J. U., Payne, G. S. Regulation of clathrin adaptor function in endocytosis: A novel role for the SAM domain. EMBO J. 29, 1033-1044 (2010).
- Kaksonen, M., Toret, C. P., Drubin, D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 123, 305-320 (2005).
- Longtine, M. S., McKenzie, A., Demarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P., Pringle, J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 14, 953-9561 (1998).
- Wach, A., Brachat, A., Alberti-Segui, C., Rebischung, C., Philippsen, P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 13, 1065-1075 (1997).
- Robinson, J. S., Klionsky, D. J., Banta, L. M., Emr, S. D. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 8, 4936-4948 (1988).
- Ito, H., Fukuda, Y., Murata, K., Kimura, A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriology. 153, 163-168 (1983).
- Vida, T. A., Emr, S. D. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 128, 779-792 (1995).
- Sikorski, R. S., Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 122, 19-27 (1989).
- Piao, H. L., Machado, I. M., Payne, G. S. NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol Biol Cell. 18, 57-65 (2007).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone