Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Decellularization and Recellularization of Whole Livers
W tym Artykule
Erratum Notice
Podsumowanie
Perfusion decellularization is a novel technique to produce whole liver scaffolds that retains the organ's extracellular matrix composition and microarchitecture. Herein, the method of preparing whole organ scaffolds using perfusion decellularization and subsequent repopulation with hepatocytes is described. Functional and transplantable liver grafts can be generated using this technique.
Streszczenie
The liver is a complex organ which requires constant perfusion for delivery of nutrients and oxygen and removal of waste in order to survive1. Efforts to recreate or mimic the liver microstructure with grounds up approach using tissue engineering and microfabrication techniques have not been successful so far due to this design challenge. In addition, synthetic biomaterials used to create scaffolds for liver tissue engineering applications have been limited in inducing tissue regeneration and repair in large part due to the lack of specific cell binding motifs that would induce the proper cell functions2. Decellularized native tissues such blood vessels3and skin4on the other hand have found many applications in tissue engineering, and have provided a practical solution to some of the challenges. The advantage of decellularized native matrix is that it retains, to an extent, the original composition, and the microstructure, hence enhancing cell attachment and reorganization5.
In this work we describe the methods to perform perfusion-decellularization of the liver, such that an intact liver bioscaffold that retains the structure of major blood vessels is obtained. Further, we describe methods to recellularize these bioscaffolds with adult primary hepatocytes, creating a liver graft that is functional in vitro, and has the vessel access necessary for transplantation in vivo.
Protokół
1. Liver Decellularization
- Harvest a rat liver with portal vein cannulation using and 18-gauge catheter. Leave the inferior and superior vena cava open. Keep the organ hydrated in phosphate buffered saline (PBS) in a 10-cm petri dish.
- Set up a perfusion system that consists of 8-liter reservoir, peristaltic pump and a bubble trap.
- Fill the perfusion system with phosphate buffered saline and keep it running for 10 minutes. Fill PBS into a 10-cm petri dish and reduce the flow rate of phosphate buffered saline to 1 mL/min.
- Carefully transfer the harvested liver to the phosphate buffered saline filled 10-cm petri dish.
- Continue with PBS perfusion overnight.
- Start perfusion with 0.01% (w/v) sodium dodecyl sulfate (SDS) in distilled water for 5 minutes.
- Perfuse with PBS for 1 hour.
- Repeat steps 1.6 and 1.7 three more times increasing the SDS perfusion time to 10, 15 and 20 minutes at each time.
- Continue perfusion with 0.01% (w/v) SDS for 24 hours.
- Continue perfusion with 0.1% (w/v) SDS for 24 hours.
- Perfuse with 0.2% (w/v) SDS for 3 hours.
- Perfuse with 0.5% (w/v) SDS for 3 hours.
- Perfuse with distilled water for 15 minutes.
- Perfuse with 1% (w/v) Triton X-100 in distilled water for 30 minutes to remove any bound nucleic acids.
- To wash the decellularized liver matrix (DLM), perfuse with PBS for 2 hours.
- Optional: Resect all lobes except the median lobe.
- Store the DLM in a clean and sealed Petri dish soaked in PBS at 4oC until ready to use (Figure 1).
- To sterilize the DLM: 1) Flush the DLM with sterile PBS containing 0.1% (v/v) peracetic acid and 4% (v/v) ethanol and incubate for 3 hours at 4oC. 2) Wash with sterile PBS 2 times. 3) Wash with sterile PBS containing 2% penicillin-streptomycin, 10ug/ mL gentamicin, and 2.5 ug/ mL amphotericin B. Store the decellularized liver in the same solution at 4oC until ready to use for recellularization experiments.
2. Recellularization of Decellularized Liver Matrix
- Set up a perfusion system that consists of a perfusion chamber, peristaltic pump and a bubble trap under sterile conditions. Fill the perfusion system with 200 mL of culture medium, e.g., high glucose DMEM (Sigma), 10% fetal bovine serum (Hyclone,), 100 U mL-1 penicillin, and 100 μg mL-1 streptomycin (Invitrogen).
- Place the decellularized liver matrix in the perfusion chamber and connect the DLM to the perfusion system through the portal vein cannula while the pump is running at 5 mL/min to avoid formation of any air bubbles.
- Allow perfusion of the medium through the DLM for 30 minutes.
- Isolate primary hepatocytes from an adult rat with at least 90% viability.
- Stop the flow in the perfusion system and slowly inject 50 million hepatocytes (in 1-3 mL of culture medium) into perfusion system through the bubble trap.
- Start the flow at 10 mL/min and recirculate the medium for 10 minutes.
- Repeat steps 2.5 and 2.6 until a total of 200 million cells are introduced into the DLM (Figure 2)6.
- Once all the cells are perfused into the DLM, collect the perfusate into four 50-ml centrifuge tubes and centrifuge at 600 rpm for 10 minutes. Discard the supernatants and collect the pellets into a single tube. Determine the number of cells and viability via trypan blue exclusion to determine the seeding efficiency.
3. In Vitro Culture of the Recellularized Liver Graft
- Set up a perfusion system that consists of a perfusion chamber, peristaltic pump, oxygenator and a bubble trap under sterile conditions (Figure 3). Fill the perfusion system with 50 mL of culture medium, e.g., Williams' E (Sigma), 5% fetal bovine serum (Hyclone,),0.5 U mL-1 insulin (Eli Lilly), 20 ng mL-1 EGF (Invitrogen), 14 ng mL-1 glucagon(Bedford Laboratories),7.5 μg mL-1 hydrocortisone (Pharmacia), 100 U mL-1 penicillin, and 100 μg mL-1 streptomycin (Invitrogen).
- Place the recellularized liver graft in the perfusion chamber and connect the DLM to the perfusion system through the portal vein cannula while the pump is running at 5 mL/min to avoid formation of any air bubbles.
- Aseptically close the perfusion chamber and seal tightly to avoid any leakage during culture.
- Transfer the perfusion system to an incubator that is 37oC and has 10% CO2 and increase the perfusion flow rate to 15 mL/min.
- Connect the oxygenator to a 95% O2 and 5% CO2 gas mixture tank and set the gas flow rate to 0.5 liters/min. This should achieve an oxygen partial pressure of approximately 400 mmHg.
- The culture may continue up to 10 days with daily changes of culture medium. The culture medium may be sampled daily for monitoring of liver functions of the graft such as albumin, urea and total bile acid secretion. At the end of the culture period, the recellularized liver graft may be sampled for molecular and histological analysis.
4. Representative Results:
The complete decellularization of a rat liver takes about 72 hours using the described protocol. The resulting matrix retains 100% of the fibrillar collagen, 50% of the glycosaminoglycans and only 5% of the DNA of the native liver (Table 1)6. The vascular structure of the matrix is preserved as evidenced by corrosion casting and scanning electron microscopy analysis (Figure 4)6. The presence of the vascular microarchitecture within the DLM facilitates its repopulation with cells with an efficiency of 96% and its subsequent perfusion for in vitro culture. The recellularized liver graft may be cultured up to 10 days in vitro and displays proper liver functions as confirmed via albumin, urea and total bile acid secretion (Figure 5)6.
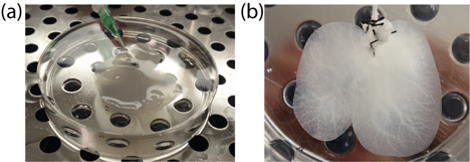
Figure 1. Decellularized liver matrix at the end of the decellularization process. (a) the whole liver (b) the median lobe after resection.

Figure 2. Schematic representation of the recellularization of the DLM.
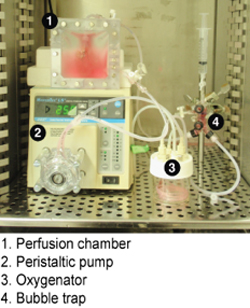
Figure 3. Perfusion system setup for in vitro culture of the recellularized liver graft.

Figure 4. The microvascular structure is retained in the decellularized liver matrix. Corrosion cast images of a) a normal liver b) a decellularized liver, portal (red) and venous (blue) vasculature. Scanning electron microscopy images of the DLM c) a vessel, d) a section featuring bile duct-like small vessels (arrows), Scale bars (a,b) 5 mm (c,d) 20 μm.

Figure 5. Liver specific functions of the recellularized liver graft during the in vitro perfusion culture. a) albumin secretion (p = 0.5249), b) urea production (p = 0.5271) and c) total bile acid secretion (p = 0.0114). Statistical analysis of the difference between the experiment and the control was done over the 10 d culture period by Friedman's test at a = 0.01. Error bars represent s.e.m. (n = 3).
| Fresh livera | Decellularized liver matrixa | p-values | % of fresh liver | |
| n = 4 | n = 8 | |||
| Collagen | 0.07 ± 0.01 | 0.08 ± 0.03 | 0.56 | 114% |
| (mg per g liver) | ||||
| Glycosaminoglycans | 73.1 ± 6.7 | 34.2 ± 2.9 | 0.004 | 47% |
| (mg per g liver) | ||||
| DNA | 14.9 ± 5.6 | 0.44 ± 0.08 | 3.3 10-5 | 2.9% |
| (mg per g liver) |
Table 1. The biochemical composition of the decellularized liver matrix compared to the native liver.
aValues are represented as mean ± s.e.m.
Dyskusje
The perfusion decellularization method described here produces a whole liver scaffold that has the same gross structure and the vascular microarchitecture of the native liver. The scaffold has an extracellular matrix composition similar to the native liver. The recellularization method achieves repopulation of the scaffold with cells at high efficiency and the cells remain viable and functional during the in vitro culture period tested. With the addition of nonparenchymal cells into the recellularized liver graf...
Ujawnienia
No conflicts of interest declared.
Podziękowania
The authors would like to thank Jack Milwid for the design of the in vitro perfusion chamber. This work was supported by grants from the US NIH, R01DK59766 and R01DK084053 to M.Y., R00DK080942 to K.U., US NSF CBET- 0853569 to K.U. and the Shriners Hospitals for Children to B.E.U. (grant no. 8503). We also acknowledge support and the Shriners Hospitals for Children.
Materiały
| Name | Company | Catalog Number | Comments |
| Sodium dodecyl sulfate | Sigma-Aldrich | L4390 | |
| Triton X-100 | Sigma-Aldrich | T8787 | |
| Masterflex L/S Digital Drive | Cole-Parmer | EW-07523-80 | |
| Masterflex L/S Standard pump head | Cole-Parmer | EW- 07013-81 | |
| Bubble trap | Radnoti Glass Technology Inc. | 130149 |
Odniesienia
- Kulig, K. M., Vacanti, J. P. Hepatic tissue engineering. Transpl Immunol. 12, 303-310 (2004).
- Lutolf, M. P., Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature biotechnology. 23, 47-55 (2005).
- Dahl, S. L., Koh, J., Prabhakar, V., Niklason, L. E. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 12, 659-666 (2003).
- Schechner, J. S. Engraftment of a vascularized human skin equivalent. FASEB J. 17, 2250-2256 (2003).
- Gilbert, T. W., Sellaro, T. L., Badylak, S. F., F, S. Decellularization of tissues and organs. Biomaterials. 27, 3675-3683 (2006).
- Uygun, B. E. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. , (2010).
Erratum
Formal Correction: Erratum: Decellularization and Recellularization of Whole Livers
Posted by JoVE Editors on 3/14/2011. Citeable Link.
A correction was made to Decellularization and Recellularization of Whole Livers. There was an error with an author's name. The author's last name had a typo and was corrected to:
Nima Saeidi
instead of:
Nima Saedi.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone