Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Dissection and Staining of Drosophila Larval Ovaries
W tym Artykule
Podsumowanie
How niches and stem cells form during development is an important question with practical implications. In the Drosophila ovary, germ line stem cells and their somatic niches form during larval development. This video demonstrates how to dissect, stain and mount female gonads from late third instar (LL3) Drosophila larvae.
Streszczenie
Many organs depend on stem cells for their development during embryogenesis and for maintenance or repair during adult life. Understanding how stem cells form, and how they interact with their environment is therefore crucial for understanding development, homeostasis and disease. The ovary of the fruit fly Drosophila melanogaster has served as an influential model for the interaction of germ line stem cells (GSCs) with their somatic support cells (niche) 1, 2. The known location of the niche and the GSCs, coupled to the ability to genetically manipulate them, has allowed researchers to elucidate a variety of interactions between stem cells and their niches 3-12.
Despite the wealth of information about mechanisms controlling GSC maintenance and differentiation, relatively little is known about how GSCs and their somatic niches form during development. About 18 somatic niches, whose cellular components include terminal filament and cap cells (Figure 1), form during the third larval instar 13-17. GSCs originate from primordial germ cells (PGCs). PGCs proliferate at early larval stages, but following the formation of the niche a subgroup of PGCs becomes GSCs 7, 16, 18, 19. Together, the somatic niche cells and the GSCs make a functional unit that produces eggs throughout the lifetime of the organism.
Many questions regarding the formation of the GSC unit remain unanswered. Processes such as coordination between precursor cells for niches and stem cell precursors, or the generation of asymmetry within PGCs as they become GSCs, can best be studied in the larva. However, a methodical study of larval ovary development is physically challenging. First, larval ovaries are small. Even at late larval stages they are only 100μm across. In addition, the ovaries are transparent and are embedded in a white fat body. Here we describe a step-by-step protocol for isolating ovaries from late third instar (LL3) Drosophila larvae, followed by staining with fluorescent antibodies. We offer some technical solutions to problems such as locating the ovaries, staining and washing tissues that do not sink, and making sure that antibodies penetrate into the tissue. This protocol can be applied to earlier larval stages and to larval testes as well.
Protokół
1. Egg laying
- Five days before dissection: allow mated females to lay eggs for 2-4 hours on fresh food supplemented with yeast. To get synchronized and well-developed larvae, it is important not to have overcrowded cultured (about 30 eggs/ 25mm vial). Typically, 7-16 females are used per vial, depending on how well they lay.
2. Selecting larvae
- Prepare a 9-well glass dissecting dish filled with Ringer's medium (128mM NaCl, 2mM Kcl, 1.8mM CaCl2, 4mM MgCl2, 35.5mM Sucrose, 5mM Hepes pH 6.9).
- Prepare cell strainers in a six-well plate containing Ringer's medium and place it on ice. Alternatively, we use specially made molds fitted with a nylon mesh.
- Pick timed larvae from vial walls using fine biological tweezers (forceps) and place them in the ringer's containing dissecting dish.
- Transfer a female larva to a clean well. We distinguish females from males by their gonads. Male testes are easily identified as big clear ovals embedded in the posterior third of the fat body. Female ovaries, located at the same part of the fat body, can be identified as a much smaller, clear, round spheres.
3. Dissection of larva
- Hold the larva down just posterior to the brain with forceps and remove the head with a second pair of forceps.
- Place the remaining posterior part on the dorsal side, with the trachea facing down.
- Hold the larva by the posterior spiracles with one pair of forceps and push it inward slowly while the other pair is sliding the cuticle posteriorly until about half the larval fat body emerges.
- Firmly hold the posterior end and use the other pair of forceps to loosely hold the cuticle. Gently and slowly pull away the posterior end so that the cuticle and the attached intestine slide through the gap. At the end of this process, the fat body should be completely separated from the intestine and the cuticle. Disconnect the intestine from the anterior part of the fat body. To make staining and mounting easier, it is important that the fat body will remain intact.
- Wet a pasture pipette thoroughly with Ringer's medium, preferably from a well containing dissected larvae. This helps coat the pipette and prevents the fat body from sticking to it. Use the pasture pipette to transfer the fat body into the ice cold Ringer's medium in the cell strainer.
4. Fixation and Staining
All steps are performed at room temperature, except for the incubation with first antibody, at 4 °C.
- Incubate the fat body in 5% formaldehyde in Ringer's medium. for 20 minutes with gentle agitation.
- Wash for 5 minutes with 1% PBT (1% Triton x-100 in PBS). Repeat this step for 10 minutes and a third time for 45 minutes. 1% Triton X-100 is required to perforate the larval ovary and allow antibodies to penetrate it.
- Block with 0.3% PBTB (0.3% Triton x-100 and 1% BSA in PBS) for 1 hour with gentle agitation
- Incubate with the desired 1st antibody diluted in 0.3% PBTB over night at 4°C with gentle agitation. This step is usually performed in 0.2 ml tubes on a roller.
- Transfer fat body back to cell strainers.
- Wash 3 times, 30 minutes each, with 0.3% PBTB with gentle agitation.
- Block with 0.3% PBTB supplemented with 5% normal donkey serum for 1 hour with gentle agitation.
- Incubate with an appropriate secondary antibody diluted (according to manufacturer's specification) in the blocking solution (0.3% PBTB supplemented with 5% normal donkey serum in 0.3% PBT) for 2 hours with gentle agitation. If the antibody is fluorescent, incubate in the dark from this point onward. This step is usually performed in 0.2 ml tubes on a roller.
- Wash 3 times for 30 minutes each with 0.3% PBT with gentle agitation.
5. Mounting
- Transfer the fat body to a clean eppendorf tube. Very carefully remove all liquids away and immediately cover with Vectashield mounting media. We use vectashield routinely since it does not harden while the ovaries are being separated from the fat body.
Samples can be stored in mounting media at 4°C for up to a week. - Cut the end of a pipette tip and use it to carefully transfer the fat body with minimal volume of mounting media (about 30 μl for a 30mm cover-slip) onto a microscope slide.
- Use two nickel plated pin holders, holding 0.1 mm diameter pins to spread out the fat body. The ovaries are located at the posterior third of the fat body. The fat body that surrounds them is usually shaped like a 'flower'. This helps identify the location of the ovaries. Dissect out the 'flowers' from the rest of the fat body and discard the latter.
- Remove the fat body surrounding the gonad by crossing the two pins carefully around it. Place the isolated gonad on the slide away from the fat body residue.
- Cover with a cover slip and seal with nail polish.
- Visualize directly using a confocal microscope. Samples can be stored at 4°C for up to three weeks.
6. Representative Results
We have used the above protocol to follow the establishment of several cell lineages within the somatic ovary, including GSCs and their somatic niches. For this purpose we use specific antibodies and markers to distinguish between the different cell types in the developing gonad. Here we show an example of two LL3 ovaries stained with different combinations of antibodies. Figure 1A highlights terminal filament and cap cells (green), which together form the somatic cells of the niche. Figure 1B, shows the Intermingled cells (ICs, magenta), which directly contact germ cells (blue).
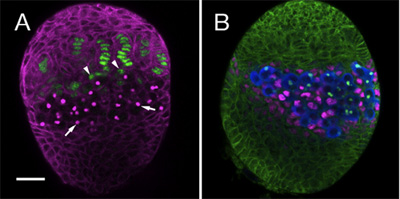
Figure 1. LL3 ovaries. (A) monoclonal antibody 1B1 (magenta) outlines somatic cells and stains the fusome, an intracellular organelle within PGCs (arrows). The enhancer trap hh-lacZ (anti β-galactosidase, green) is expressed in terminal filaments. At the base of the filament somatic cap cells (arrowheads) can be observed. (B) 1B1 antibody (green) outlines all somatic cells in the ovary. Anti-Vasa (blue) labels all PGCs. PGCs directly contact the Intermingled cells (ICs, anti-traffic jam, magenta). Bar (for A and B) is 20 μm.
Dyskusje
This video demonstrates an isolation and staining protocol of late third instar larval ovaries. To perform this protocol routinely and reliably, attention should be paid to the following points:
- For synchronized and well-developed larvae, over crowding must be avoided.
- To avoid loss of the small translucent ovaries, make sure to dissect the fat body intact. This will also help in locating the ovaries at the mounting stage, particularly when mounting younger (second or beginning of third instar)...
Ujawnienia
No conflicts of interest declared.
Podziękowania
IM is supported by the Marie Curie re-integration grant. This work was supported by the Israel Science Fund Grant no. 1146/08, by the Helen and Martin Kimmel Institute for Stem Cell Research at the Weizmann Institute of Science and the Leir Charitable Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| NaCl | JT Baker | ||
| Kcl | Merck & Co., Inc. | ||
| CaCl2 | Sigma-Aldrich | ||
| MgCl2 | Merck & Co., Inc. | ||
| Sucrose | JT Baker | ||
| Hepes | Sigma-Aldrich | ||
| PBS | Sigma-Aldrich | ||
| Triton X-100 | Sigma-Aldrich | ||
| Albumin Bovine Fraction V | MP Biomedicals | 160069 | |
| Dumont biology tweezers 5 dumstar polished | Fine Science Tools | 11295-10 | |
| Nickel plated pin holder | Fine Science Tools | 26018-17 | |
| s.s minutien pins 0.1mm diam, 10mm long | Fine Science Tools | 26002-10 | |
| 9 well plates 85X100 mm, 22mm o.d.x7mm deep | Corning | 7220-85 | |
| Stereo Microscope MZ 16.5 with a standard transmitted light base TL ST | Leica Microsystems | ||
| 6 well plates | Costar | 3516 | |
| Slides | Menzel-Glaser | 798 | |
| Cover slips | Corning | 2940-223 | |
| Mounting media | Vectashield | H-1200 | |
| Cell strainer | Falcon BD | FAL352350 | |
| 1B1 antibody | Developmental Studies Hybridoma Bank | ||
| Anti-Traffic Jam | Laboratory of Dr. Dorothea Godt | ||
| Anti-Vasa | Laboratory of Dr. Ruth Lehmann | ||
| Anti β-Galactosidase | Cappel | ||
| Secondary Antibodies | Jackson ImmunoResearch |
Odniesienia
- Fuller, M. T., Spradling, A. C. Male and female Drosophila germline stem cells: two versions of immortality. Science. 316, 402-404 (2007).
- Li, L., Xie, T. Stem cell niche: structure and function. Annual review of cell and developmental biology. 21, 605-631 (2005).
- Chen, D., McKearin, D. Gene circuitry controlling a stem cell niche. Curr Biol. 15, 179-184 (2005).
- Kai, T., Spradling, A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proceedings of the National Academy of Sciences of the United States of America. 100, 4633-4638 (2003).
- Kai, T., Spradling, A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 428, 564-569 (2004).
- Lopez-Onieva, L., Fernandez-Minan, A., Gonzalez-Reyes, A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 135, 533-540 (2008).
- Song, X., Zhu, C. H., Doan, C., Xie, T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 296, 1855-1857 (2002).
- Tazuke, S. I. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development. 129, 2529-2539 (2002).
- Xie, T., Spradling, A. C. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 94, 251-260 (1998).
- Xie, T., Spradling, A. C. A niche maintaining germ line stem cells in the Drosophila ovary. Science. , 290-328 (2000).
- Guo, Z., Wang, Z. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 136, 3627-3635 (2009).
- Wang, X., Harris, R. E., Bayston, L. J., Ashe, H. L. Type IV collagens regulate BMP signalling in Drosophila. Nature. 455, 72-77 (2008).
- Godt, D., Laski, F. A. Mechanisms of cell rearrangement and cell recruitment in Drosophila ovary morphogenesis and the requirement of bric a brac. Development. 121, 173-187 (1995).
- Sahut-Barnola, I., Godt, D., Laski, F. A., Couderc, J. L. Drosophila ovary morphogenesis: analysis of terminal filament formation and identification of a gene required for this process. Developmental biology. 170, 127-135 (1995).
- Song, X., Call, G. B., Kirilly, D., Xie, T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. 134, 1071-1080 (2007).
- Zhu, C. H., Xie, T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development. 130, 2579-2588 (2003).
- Ward, E. J. Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr Biol. 16, 2352-2358 (2006).
- Gilboa, L., Lehmann, R. Repression of primordial germ cell differentiation parallels germ line stem cell maintenance. Curr Biol. 14, 981-986 (2004).
- Gilboa, L., Lehmann, R. Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature. 443, 97-100 (2006).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone