Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Mammary Transplantation of Stromal Cells and Carcinoma Cells in C57BL/6J Mice
W tym Artykule
Podsumowanie
In this report, we demonstrate a system to isolate and culture donor cells from the mouse mammary gland, and orthotopically transplant these cells in recipient mice to analyze stromal: epithelial interactions during mammary tumor development.
Streszczenie
The influence of stromal cells, including fibroblasts on mammary tumor progression has been well documented through the use of mouse models, in particular through transplantation of stromal cells and epithelial cells in the mammary gland of mice. Current transplantation models often involve the use of immunocompromised mice due to the different genetic backgrounds of stromal cells and epithelial cells. Extracellular matrices are often used to embed the two different cell types for consistent cell-cell interactions, but involve the use of Matrigel or rat tail collagen, which are immunogenic substrates. The lack of functional T cells from immunocompromised mice prevents accurate assessment of stromal cells on mammary tumor progression in vivo, with important implications on drug development and efficacy. Moreover, immunocompromised mice are costly, hard to breed and require special care conditions. To overcome these obstacles, we have developed an approach to orthotopically transplant stromal cell and epithelial cells into mice from the same genetic background to induce consistent tumor formation. This system involves harvesting normal, carcinoma associated fibroblasts, PyVmT mammary carcinoma cells and collagen from donor C57BL/6J mice. The cells are then embedded in collagen and transplanted in the inguinal mammary glands of female C57BL/6J mice. Transplantation of PyVmT cells alone form palpable tumors 30-40 days post transplantation. Endpoint analysis at 60 days indicates that co-transplantation with fibroblasts enhances mammary tumor growth compared to PyVmT cells transplanted alone. While cells and matrix from C57BL/6J mice were used in these studies, the isolation of cells and matrix and transplantation approach may be applied towards mice from different genetic backgrounds demonstrating versatility. In summary, this system may be used to investigate molecular interactions between stromal cells and epithelial cells, and overcomes critical limitations in immunocompromised mouse models.
Protokół
1. Isolation and extraction of donor collagen from C57BL/6J mice
- Sacrifice mature normal female C57BL/6J mice using approved IACUC methods.
- Harvest the tails and soak in 70% ethanol for 45 minutes to sterilize tissues. Dry the tails with tissue paper, wrap in aluminum foil. Store the tails in -20°C until needed.
- Place the tails in a sterile environment such as a laminar flow hood. Using scissors, split the skin at the tail root and peel away from the tail. Remove 0.5 to 1 cm from both ends of the tail and divide the remaining tail up into three or four pieces. Dissect the tendons from the tails and separate the tendons into individual fibers using a scalpel or razor blade.
- Transfer the tendon fibers to a sterile container and wash with sterile distilled water. Transfer tendons from tail of 5-7 mice to a 50 ml conical tube containing 35 ml sterile de-ionized water containing 50 units/ml penicillin penicillin, 50 μg/ml streptomycin and 250 ng/ml amphotericin, and 0.034 N acetic acid diluted from a stock solution of glacial acetic acid (17 N). Place on rocker at 4°C for a week to extract the collagen.
- Spin down debris in a table top centrifuge at 3000 g for 15 minutes. Transfer supernatant, approximately 30 mls to two polycarbonate tubes adapted for the Beckman 50.2 Ti rotor and spin at 35,000 g (approximately 17000 rpm) for 1 hour.
- The volume of the supernatant will need to be reduced to achieve a concentration of 1-2 mg/ml. Transfer the supernatant to an ultracel 50 k Amicon filtration unit and spin in a table top centrifuge at 3000 rpm for 15 at 4°C. Transfer the filtrate to a sterile conical tube and save. Repeat the centrifugation until the volume is reduced to 5-6 ml.
- Read the protein concentration. We use a standard Bradford assay (Biorad), using BSA standards (Biorad) and undiluted samples. Measure a sample from your filtrate, as a negative control.
- Further check for purity of extraction by resolving a sample of purified collagen on a 6% SDS polyacrylamide gel, 10 lanes, 1.5 mm gel thickness. Prepare 20 μg of sample in 30 μl 1x SDS-PAGE loading buffer containing 63 mM Tris HCl 10% glycerol, 2% SDS 0.0025% bromophenol blue in 1.5 ml eppendorf tubes. Prepare an equivalent amount of protein from rat tail collagen type I as a positive control. In addition, prepare a set of BSA standards at 2.5, 5, 10, 20 μg diluted in PBS and 1X loading buffer. Boil the samples at 95°C for 5 minutes.
- Let the samples cool and briefly microfuge (maximum speed for 10 seconds) to spin down condensation.
- Load the samples samples along with a molecular weight marker. For example, load 5 μl of Kaleidoscope Biorad protein standard. Electrophorese at 150 volts for approximately 1 hour or until the dye front reaches the bottom.
- Remove the gel and stain in coomassie blue buffer. Prepare this buffer by mixing 2 g of coomassie blue, 75 ml glacial acetic acid, 500 ml ethanol in a total volume of 1000 ml containing de-ionized water. Also prepare the same amount of destain buffer using the recipe except without coomassie blue reagent. Cover the gel with a sufficient volume for coomassie blue and place on a rocker.
- Rock gently for 1 hour at RT and decant the stain. Replace the destain solution when it turns dark blue. Detain the gel overnight or until the individual bands are resolved on the gel. Locate two collagen bands (molecular weights= 90 kda and 130 kda) and note the strength of the bands against the standards and controls. Image the gel using a gel doc system, if necessary.
2. Isolation and culture of donor mammary carcinoma cells and fibroblasts from normal and PyVmT C57BL/6J mice
- Sacrifice age matched female normal or PyVmT transgenic mice after 10 weeks of age when tumors are evident and palpable in PyVmT transgenic mice using approved IACUC methods.
- Expose the mouse on its back on a flat surface and immobilize the limbs using tape adhesive.
- Make an upside down T incision between the nipples of the thoracic and inguinal mammary gland and pull the skin flap back to expose the mammary glands. Remove the mammary tissues and mince into small pieces using surgical scissors.
- Prepare 100 ml of an enzymatic digestion cocktail containing: 200 mg trypsin, 500 mg collagenase, 4 mg DNAse, 100,000 units hyaluronidase in sterile PBS and antibiotics overnight on ice and then for 3 h at 37°C.
- Add 10 ml of PBS containing 10% fetal bovine serum (FBS) to each sample and centrifuge cell pellet in a table top centrifuge at 1500 rpm for 5 minutes at 4°C.
- Gently aspirate the supernatant and resuspend the cell pellet in 5-7 ml of PBS/10% FBS. Repeat the spin and wash two more times.
- Resuspend the cell pellet in 10 ml of DMEM containing 10% FBS containing 50 units/ml penicillin , 50 μg/ml streptomycin and 250 ng/ml amphotericin on 10 cm dishes coated with collagen type I. To coat plates with collagen, dilute the collagen 1 ml (using stock 1.5 mg/ml) into 39 ml of 0.02 N acetic acid diluted in sterile distilled water or PBS. Pipet 5 mls of collagen working solution onto 10 cm plates. Incubate at room temperature for a minimum for 10 minutes. Aspirate excess collagen. Parafilm unused plates and store at 4°C for a week.
- Change the media 2- 3 times a week. Successful digestion of tissues will be characterized by the presence of epithelial foci surrounded by spindle shaped fibroblastic cells.
- Separate the fibroblasts and epithelial cells by selective tryspinization. Aspirate the media and wash the cells with PBS once. Pipet 1 ml of 0.25% trypsin/0.54 mM EDTA onto cells and incubate at room temperature. Fibroblasts are more loosely adherent than epithelial cells. Check for fibroblast detachment under the microscope after 2 minutes; cells should be floating in suspension or rounded up while epithelial foci should still be adhered to the surface.
- Lightly tap the dish to loosen the fibroblasts and gently pipet 10 mls of serum containing medium in the dish to remove the fibroblasts.
- Pipet the media containing fibroblasts in a 15 ml conical tube and centrifuge as described in step 5). Replate these cells onto collagen coated 10 cm dishes and repeat the selective trypsinization process until fibroblasts and epithelial cells are completely separated.
- Check for cell purity by immunofluorescence staining for epithelial markers such as CK14 and CK18 and fibroblast markers such as alpha smooth muscle actin (α-sma).
3. Immunofluorescence staining of cultured cells
- Place glass coverslips in 6 cm dishes. Sterilize by UV exposure for 5 to 10 minutes in a laminar flow hood.
- Coat the coverslip with 1-2 ml of working stock collagen solution for 10 minutes at room temperature (RT). Aspirate excess.
- Trypsinize and plate 200,000 epithelial cells or fibroblasts per sample. Add 3-5 ml of complete media and incubate for 24 hours.
- Aspirate the media, wash cells with PBS and fix in ice cold methanol at -20°C for 7 minutes.
- Wash cells with PBS twice to remove ethanol, and block with 1-2 ml of PBS containing 1% FBS for 1 hour at RT.
- Using forceps, remove the coverslips and place in a large shallow container covered with parafilm or other hydrophobic surface.
- Dilute the following primary antibodies 1:100 in blocking solution: anti-smooth muscle actin (α-sma, anti-CK14 (basal epithelial marker), anti-CK18 (luminal epithelial marker).
- Pipet 100 μl of antibodies directly onto the coverslips for 1 hour at RT. Incubate a separate coverslip of cells in blocking solution; this sample will serve as the secondary antibody control.
- Aspirate antibody and pipet 1 ml of PBS directly on the coverslip to wash. Aspirate and repeat 3-5 times.
- Dilute the following secondary antibodies in blocking solution: anti-mouse-alexa 488 at 1:100, anti-mouse biotinylated at 1: 500, anti-mouse-alexa-568.
- Wrap the container with the coverslips in aluminum foil. Pipet 100 μl of appropriate secondary antibody directly on the coverslips: α-sma with anti-mouse-alexa 568, CK 14 staining with anti-mouse biotinylated and CK18 with anti-mouse-alexa 488. Cover the container to protect the samples from visible light.
- Aspirate antibody and wash with PBS as in step 9). Leave the samples in PBS, except for CK14 staining.
- Dilute streptavidin conjugated to alexa 568 1:500 in PBS. Incubate the CK14 stained samples with strepatividin-alexa 568 for 30 minutes at RT in the dark. Wash in PBS as in step 9)
- Dilute DAPI 1: 500 in PBS. Aspirate the PBS from the samples and incubate with DAPI for 10 minutes RT in the dark.
- Rinse samples with PBS. Pipet 100 μl of Prolong anti-fade reagent onto glass slides, turn the coverslip over so that the cells are facing the glass slide. Tilt the coverslip to one angle to gently make contact with the mounting media.
- Image the samples using an immunofluorescence microscope. Keep the samples in the dark to protect the immunofluorescence signal, which will last approximately 2 weeks.
4. Preparation of collagen embedded cells for grafting
- To embed cells in collagen, it is necessary to lower the pH of the collagen to achieve polymerization by mixing the collagen with a setting solution. Prepare the setting solution by mixing 100 ml of 10X Earl's Balanced Salt Solution (EBSS) with 2.45 g of NaHCO3, 7.5 ml of 1 M NAOH and 42.5 ml of sterile distilled water. Further sterilize using a bottle top 0.22 micron filter unit attached to a sterile bottle.
- Mix the collagen (stock concentration 1 mg/ml) with setting solution at a starting 4: 1 ratio. For one graft, mix 100 μl of collagen with 25 μl of setting solution in an eppendorf tube. Vortex briefly or pipet the sample up and down to mix the sample thoroughly.
- Add collagen or setting solution at 5-10 μl increments and mix thoroughly until the phenol red dye in the mixture changes to a light pink to orange color, reflecting a neutral pH. A yellow color indicates acidic pH while dark pink reflects basic pH. Keep the mixture on ice to prevent polymerization.
- Remove the fibroblasts and epithelial cells from the plate by trypsinization. First, aspirate the media and wash with 5 ml of PBS. Pipet 1 ml of 0.25% trypsin/0.54 mM EDTA in HBSS per plate. Incubate fibroblasts at RT for 2-5 minutes. Incubate epithelial cells at 37oC for 2-6 minutes. Tap the plates gently to loosen the cells. Check for detachment using a microscope.Quench the trypsin by pipetting 9 ml of complete medium on the plate and transfer the cells to a sterile 15 ml conical tube.
- Remove 50 ul and count the cells by hemocytometer. Note the total volume of cells in each tube (approximately 10 ml).
- Pellet the cells at 1500 rpm for 5 min at RT. Aspirate the cell pellet and resuspend the cells to a concentration of 100,000 cells/100 μl. For example, resuspend 500,000 cells in a total volume of 500 μl of complete media.
- Mix 250,000 stromal cells (250 μl) and 100,000 tumor cells (100 μl) in a separate tube. Microfuge 10 sec 1000 rpm, remove supernatant by pipetting. Add 50 μl of the adjusted collagen solution to cells. Pipet up and down to disperse the cells evenly. Pipet the 50 μl in a sterile 6 cm tissue culture plate to form a circular drop. Incubate the graft at 37°C for 10 minutes to polymerize.
- Gently add 5 mls of complete media in the plate by tilting the plate at an angle and slowly pipetting the media by one side of the plate. Tilt the plate around until the media covers the graft.
- Incubate for 24 hours at 37°C. Transplant immediately.
5. Orthoptic transplantation of collagen embedded cells in C57BL/6J mice
- Fabricate a thin glass rod for the transplantation surgery. Heat a glass Pasteur pipet using a Bunsen burner. Using forceps, stretch the end of the pipet out to 2-3 mm in thickness. Let cool, and sterilize by 70% ethanol.
- Sterilize the following surgical instruments by autoclaving and 70% ethanol: fine surgical scissors, blunt surgical scissors, blunt forceps, thin forceps, wound clips, wound staple
- Anesthetize mouse using with 2-3% isoflurane using an isoflurane scavenging machine. The average O2 flow rate used is 1 L/ minute through the nose cone.
- Lay the mouse on its back and immobilize the limbs with tape or other removable adhesive. Remove the hair with chemical hair remover such as Nair. Sterilize the abdomen with cotton swabbing alternatively between 70% ethanol and betadine in a target-like fashion.
- Hold up a part of the skin by the mammary gland using blunt forceps with one hand. With the other hand, using blunt surgical scissors to make an upside down T- incision between the #9 and #10 nipples or #4 and #5 nipples of the inguinal mammary glands to expose the mammary glands.
- Make a pocket incision under the lymph node or mammary artery with small surgical spring scissors.
- Remove the collagen graft from the tissue culture dish using forceps. Remove excess liquid from the collagen graft by gently dabbing onto a sterile wipe and slide the collagen graft completely into the pocket using a thin glass rod.
- Suture the skin flap using #2 gut absorbable sutures or wound staples.
- Let the mouse recover in the cage.
- Monitor the mice twice weekly at a minimum, palpating for tumors. Sacrifice the mice when the tumors reach 1 cm in diameter. Harvest tissues for analysis.
6. Representative Results:
Isolation and extraction of collagen from C57BL/6J mice
These procedures were adapted from1. Extraction of collagen protein from 5-7 mouse tails yields approximately 1-1.5 mg/ml in a 6 ml final volume, or 6- 9 mg of protein. By coommassie stain, bands corresponding to 90 kda and 130 kda are detected in the lanes loaded with samples extracted from mouse tails, indicating the presence of collagen type I and pro-collagen respectively (Figure 1).
Isolation and culture of mammary carcinoma cells and fibroblasts from normal and PyVmT C57BL/6J mice.
The procedures were adapted from2. PyVmT carcinoma cells and fibroblasts can be distinguished by differences in cell morphology and expression of specific epithelial and mesenchymal markers. PyVmT carcinoma cells are identified by cobblestone shape and co-express CK18, a luminal epithelial marker and CK14, a basal epithelial marker, but not express α-sma (Figure 2). Mammary fibroblasts are larger cells with a spindle shaped phenotype and express high levels of α-sma but do not express CK14 or CK 18 (Figure 2). These data indicate over 95% cell purity for fibroblasts and epithelial cells using the outlined procedures.
Orthoptic transplantation of collagen embedded cells in C57BL/6J mice
These procedures were adapted from3, 4. Transplantation recipient mice are sacrificed when tumors in either experimental group reach 1.0 cm in diameter, or approximately 60 days. While transplantation of PyVmT cells alone results in palpable tumors after 30- 40 days, reaching a mean tumor mass of 0.335 grams at 60 days, co-transplantation of PyVmT carcinoma cells with mammary fibroblasts results in a mean tumor mass of 0.630 grams, indicating enhancement of tumor growth by fibroblasts (Figure 3).
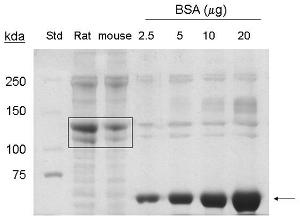
Figure 1. Coomassie stain analysis of collagen type I extraction from mouse tails. BSA (˜ 66 kda) is indicated by arrow. Commercial rat tail collagen (20 μg protein), and purified mouse tail collagen (20 μg protein) are indicated by box (˜130 kda, ˜ 90 kda proteins). Std= molecular weight standard.
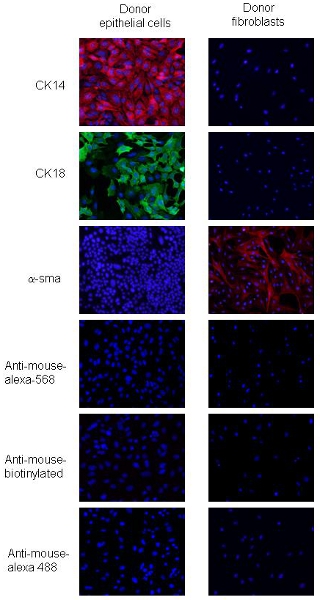
Figure 2. Immunofluorescence staining of donor PyVmT mammary carcinoma cells and fibroblasts. Panels a and b represent PyVmT mammary carcinoma cells immunostained for antibodies to CK14 and CK18. Panel c represents fibroblasts stained with antibodies to α-sma. Images shown with DAPI overlay at 20x magnification.
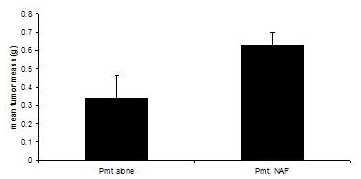
Figure 3. Mammary tumor development in C57BL/6J mice in the presence or absence of fibroblasts. Mammary tumors were harvested from mice transplanted with PyVmT carcinoma cells in the presence or absence of mammary fibroblasts and weighed. Mean+standard error of the mean. N=6 per group.
Dyskusje
The functional contribution of fibroblasts in tumor progression has been demonstrated through transplantation models, in which carcinoma associated fibroblasts co-transplanted with benign mammary epithelial cells results in increased tumor growth and invasiveness5. Conventional transplantation approaches have involved the use of SCID or nude mice to co-transplant stromal and epithelial cells from different genetic mouse backgrounds or different species. Immunocompromised mice lack functional T cells, which pl...
Ujawnienia
Animal experiments:
Experiments on animals were performed in accordance with the guidelines and regulations set forth by the IACUC committee at the University of Kansas Medical Center.
Podziękowania
This project was funded through by NIH/NCI grant number R00 CA127357 and University of Kansas Cancer Center Endowment.
Materiały
| Name | Company | Catalog Number | Comments |
| C57BL/6N mice | Harlan Laboratories | N/A | |
| MMTV-PyVmT transgenic mice | Jackson Laboratory | 002374 | |
| Fetal Bovine Serum | Fisher Scientific | SH3039603PR | |
| DMEM | VWR international | 10000113873 | |
| Penicillin/streptomycin | Fisher Scientific | MT-30-001 | |
| amphotericin | Fisher Scientific | BP2645-20 | |
| Amicon filtration columns ultracel 50k | EMD Millipore | UFC905008 | |
| Tubes for Beckman TI rotor | Beckman Coulter Inc. | 355618 | |
| Rat tail collagen | Fisher Scientific | CB 40236 | |
| 10x EBSS | Sigma-Aldrich | E7510-100ML | |
| Trypsin 1X, 0.25% in HBSS w/o Calcium and Magnesium | Fisher Scientific | MT-25-050-CI | |
| Glacial acetic acid | Fisher Scientific | A491-212 | |
| Coomasie blue | Fisher Scientific | BP101 25 | |
| Trypsin | Sigma-Aldrich | T3924-100ml | |
| Collagenase A | Sigma-Aldrich | ||
| hyalronidase | Sigma-Aldrich | H3884 | |
| DNase | Sigma-Aldrich | D5025 | |
| Kaleidoscope Protein standard | Bio-Rad | 1610375 | |
| Glass slides | Fisher Scientific | 12545-78 | |
| Glass coverslips | VWR international | 101400-042 | |
| Vimentin antibody S-20 | Santa Cruz Biotechnology, Inc. | SC-7558 | |
| α-smooth muscle actin antibody | Abcam | ab5694 | |
| CK14 antibody | Santa Cruz Biotechnology, Inc. | sc-53253 | |
| CK18 antibody | Abcam | ab668 | |
| DAPI | Sigma-Aldrich | D9542 | |
| Anti-mouse biotinylated | Vector Laboratories | BA9200 | Distributed through Fisher |
| Anti-mouse-alexa-568 | Invitrogen | A10037 | |
| Anti-mouse- alexa-488 | Invitrogen | A11001 | |
| Streptavidin- alexa-488 | Invitrogen | S11226 | |
| DAPI | Invitrogen | D21490 | |
| Prolong antifade | Invitrogen | P-36930 | |
| Surgical scissors | Fine Science Tools | 91400-12 | |
| Fine spring scissors | Fine Science Tools | 15000-02 | |
| Blunt forceps | Fine Science Tools | 11002-12 | |
| # 5 fine forceps | Fine Science Tools | 11251-10 | |
| Gut chromic suture | Fisher Scientific | NC9326254 | |
| Glass Pasteur pipet | Fisher Scientific | 22-042-815 | |
| Ethanol | Fisher Scientific | A406P 4 | |
| betadine | Fisher Scientific | NC9386574 | |
| Wound clips | Fisher Scientific | 12032-07 | |
| Wound staple | Fisher Scientific | 12031-07 |
Odniesienia
- Hayward, S., Haughney, P. C., Rosen, M. A., Greulich, K. M., Weier, H. U., Dahiya, R., Cunha, G. R. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 63, 131-131 (1998).
- Cunha, G., Hom, Y. K., Young, P., Brody, J., Asch, B. B., Ip, M. M. . Methods in Mammary Gland Biology. , (2000).
- Ethier, S. P., Ammerman, C. A., Dziubinski, M. L., Asch, B. B., Ip, M. M. . Methods in Mammary Gland Biology. , (2000).
- Medina, D., Kittrell, F., Asch, B. B., Ip, M. M. . Methods in Mammary Gland Biology. , (2000).
- Kalluri, R., Zeisberg, M. Fibroblasts in cancer. Nat Rev Cancer. 6, 392-401 (2006).
- DeNardo, D. G., Johansson, M., Coussens, L. M. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis. 27, 11-18 (2008).
- Naito, M. Macrophage differentiation and function in health and disease. Pathol Int. 58, 143-155 (2008).
- Firestein, G. S. The T cell cometh: interplay between adaptive immunity and cytokine networks in rheumatoid arthritis. J Clin Invest. 114, 471-474 (2004).
- Cheng, N., Chytil, A., Shyr, Y., Joly, A., Moses, H. L. Enhanced Hepatocyte Growth Factor Signaling by Type II Transforming Growth Factor-{beta} Receptor Knockout Fibroblasts Promotes Mammary Tumorigenesis. Cancer Res. 67, 4869-4877 (2007).
- Qiu, T. H. Global expression profiling identifies signatures of tumor virulence in MMTV-PyMT-transgenic mice: correlation to human disease. Cancer Res. 64, 5973-5981 (2004).
- Schaffhausen, B. S., Roberts, T. M. Lessons from polyoma middle T antigen on signaling and transformation: A DNA tumor virus contribution to the war on cancer. Virology. 384, 304-316 (2009).
- Cepko, C. L. Immortalization of neural cells via retrovirus-mediated oncogene transduction. Annu Rev Neurosci. 12, 47-65 (1989).
- O'Hare, M. J. Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc Natl Acad Sci U S A. 98, 646-651 (2001).
- Shay, J. W., Wright, W. E., Werbin, H. Defining the molecular mechanisms of human cell immortalization. Biochim Biophys Acta. 1072, 1-7 (1991).
- Gudjonsson, T., Villadsen, R., Ronnov-Jessen, L., Petersen, O. W. Immortalization protocols used in cell culture models of human breast morphogenesis. Cell Mol Life Sci. 61, 2523-2534 (2004).
- Raschke, W. C., Baird, S., Ralph, P., Nakoinz, I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 15, 261-267 (1978).
- Shen, G. Immortalization of endothelial cells differentiated from mouse embryonic stem cells. Shi Yan Sheng Wu Xue Bao. 35, 218-228 (2002).
- Wang, S. J., Greer, P., Auerbach, R. Isolation and propagation of yolk-sac-derived endothelial cells from a hypervascular transgenic mouse expressing a gain-of-function fps/fes proto-oncogene. In Vitro Cell Dev Biol Anim. 32, 292-299 (1996).
- Tiede, B. J., Owens, L. A., Li, F., DeCoste, C., Kang, Y. A novel mouse model for non-invasive single marker tracking of mammary stem cells in vivo reveals stem cell dynamics throughout pregnancy. PLoS One. 4, e8035-e8035 (2009).
- Guzman, R. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 39, 1300-1306 (2008).
- Duda, D. G. Differential transplantability of tumor-associated stromal cells. Cancer Res. 64, 5920-5924 (2004).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone