Method Article
Live Cell Calcium Imaging Combined with siRNA Mediated Gene Silencing Identifies Ca2+ Leak Channels in the ER Membrane and their Regulatory Mechanisms
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
The endoplasmic reticulum plays a key role in protein biogenesis and in calcium homeostasis. We have established an experimental system that allows us to address the role of Ca2+ leak channels and to characterize their putative regulatory mechanisms. This system involves siRNA mediated gene silencing and live cell Ca2+ imaging.
Streszczenie
In mammalian cells, the endoplasmic reticulum (ER) plays a key role in protein biogenesis as well as in calcium signalling1. The heterotrimeric Sec61 complex in the ER membrane provides an aqueous path for newly-synthesized polypeptides into the lumen of the ER. Recent work from various laboratories suggested that this heterotrimeric complex may also form transient Ca2+ leak channels2-8. The key observation for this notion was that release of nascent polypeptides from the ribosome and Sec61 complex by puromycin leads to transient release of Ca2+ from the ER. Furthermore, it had been observed in vitro that the ER luminal protein BiP is involved in preventing ion permeability at the level of the Sec61 complex9,10. We have established an experimental system that allows us to directly address the role of the Sec61 complex as potential Ca2+ leak channel and to characterize its putative regulatory mechanisms11-13. This system combines siRNA mediated gene silencing and live cell Ca2+ imaging13. Cells are treated with siRNAs that are directed against the coding and untranslated region (UTR), respectively, of the SEC61A1 gene or a negative control siRNA. In complementation analysis, the cells are co-transfected with an IRES-GFP vector that allows the siRNA-resistant expression of the wildtype SEC61A1 gene. Then the cells are loaded with the ratiometric Ca2+-indicator FURA-2 to monitor simultaneously changes in the cytosolic Ca2+ concentration in a number of cells via a fluorescence microscope. The continuous measurement of cytosolic Ca2+ also allows the evaluation of the impact of various agents, such as puromycin, small molecule inhibitors, and thapsigargin on Ca2+ leakage. This experimental system gives us the unique opportunities to i) evaluate the contribution of different ER membrane proteins to passive Ca2+ efflux from the ER in various cell types, ii) characterize the proteins and mechanisms that limit this passive Ca2+ efflux, and iii) study the effects of disease linked mutations in the relevant components.
Protokół
1. Preparation of stock solutions
- Prepare calcium-free buffer (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.5 mM EGTA, 10 mM glucose in 10 mM HEPES-KOH, (pH 7.35)).
- Solubilize FURA-2 AM in DMSO to obtain an 1 mM stock solution. Mix using a vortex until the solution is homogenously light yellow. Protect cup from light. Dilute the FURA-2 AM stock solution into 1 ml Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin to a final concentration of 4 μM for loading of HeLa cells.
- Prepare a 12.5 mM puromycin stock solution in distilled water (pH 7.0). Store aliquots at -20°C. Dilute the 12.5 mM puromycin stock solution in the calcium-free buffer to a final concentration of 500 μM puromycin.
- Dissolve thapsigargin in DMSO to obtain a 1 mM Stock solution.
- Solubilize 1 mg ophiobolin A in 20 μl chloroform and, subsequently, mix with 25 μl DMSO. The microcentrifuge tube remains open until chloroform is evaporated. Thus the final ophiobolin A concentration is 100 mM. Store aliquots at -20 °C. Prior to use, dilute stock solutions into calcium-free buffer to a final concentration of 100 μM. Thus, final DMSO concentration for live cell imaging does not exceed 0.1%.
- Dissolve trifluoperazine in DMSO to a concentration of 10 mM and store aliquots at -20 °C. Dilute stock solutions into calcium-free buffer to a final concentration of 10 μM prior to use. Thus final DMSO concentration for live cell imaging, like for ophiobolin A, does not exceed 0.1%.
- Dissolve the siRNAs (SEC61A1 siRNA, SEC61A1-UTR siRNA and control siRNA) in RNase free water to prepare a 20 μM stock solution. Mix using a vortex and observe solubilization by eye. Store 50 μl aliquots at -20 °C.
2. Gene silencing in HeLa cells
In order to study the contribution of a certain protein to ER Ca2+ efflux, the respective gene has to be efficiently silenced with two different siRNAs (Fig. 1). In addition, the effect of silencing has to be overcome by expression of the respective wild type gene. Typically we use siRNAs that are directed against the coding and non-coding (UTR) region, respectively, of the gene of interest. Employing UTR-directed siRNA provides a convenient way for complementation.
- Seed 5.2 x 105 HeLa cells (ATCC no. CCL-2) in a 6 cm culture dish with a 25 mm cover slip (pre-treated with 200 μl of poly-L-lysine (1 mg/ml) for 1 h) in Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin and incubate at 37°C in a humidified environment with 5% CO2 (final volume 3.9 ml).
- Transfect the cells with SEC61A1 siRNA, SEC61A1-UTR siRNA, or a negative control siRNA using HiPerFect Reagent according to the manufacturer’s instructions (final concentration of siRNA: 20 nM). Briefly, prepare siRNAs freshly in a separate microcentrifuge tube prior to the actual transfection procedure: Add 20 μl HiPerFect transfection reagent to 4 μl of each siRNA (20 μM) that is dissolved in 80 μl of OptiMEM. This mix is gently vortexed and incubated at room temperature for 10 min. Add the siRNA mix (104 μl) dropwise to the seeded 5.2 x 105 HeLa cells (3.9 ml).
- After 24 h, change the medium (3.9 ml) and transfect the cells for a second time with the same siRNA mix (104 μl).
- Evaluate silencing by Western blot analysis. It should be above 80%. Wash cells from the cell culture dish in phosphate-buffered saline (PBS) and harvest them in DMEM. Count the cells by employing the Countess Automated Cell Counter. Lyse the cells in cell lysis buffer (10 mM NaCl, 10 mM Tris-HCl, pH 8.0, 3 mM MgCl2, 0,5% NP40) supplemented with a protease-inhibitor cocktail (3 μg/ml Pepstatin A, 3 μg/ml Leupeptin, 3 μg/ml Antipain, 3 μg/ml Chymastatin) and mix with SDS-sample buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% Glycerol, 5% β-mercaptoethanol, 0.01% bromphenol blue) to obtain a final concentration of 10.000 cells/μl. Heat samples at 56 °C for 15 min and, subsequently, add some glas beads and vortex for 20 min to fragment DNA. Separate 20 μl of each sample by a Laemmli-type SDS-PAGE (typically 15% polyacrylamid for the separating gel). Transfer separated proteins onto a PVDF membrane by electroblotting (“wet-blot”) at constant 400 mA for 3 h or overnight in transfer buffer (100 mM Glycin, 12.5 mM Tris-HCl). Afterwards, block with 5% (w/v) low fat dried milk in PBS for 30 min at room temperature and then expose the blot to the primary antibody directed against the C-terminus of Sec61α from rabbit and an anti-β-actin-antibody from mouse. Wash the blot in PBS-TritonX-100 (0.05%) and twice in PBS for 5 min, respectively. Visualize the primary antibodies using ECL Plex goat-anti-rabbit IgG-Cy5- or ECL Plex goat-anti-mouse IgG-Cy3-conjugate and the Typhoon-Trio imaging system in combination with the Image Quant TL software 7.0. In the case of the SEC61A1 gene, the maximum silencing effect is typically seen 96 h after the first transfection. A representative experiment is shown in Fig. 2.
3. Complementation of HeLa cells
In order to rescue the phenotype of SEC61A1 silencing, the SEC61A1 cDNA was inserted into the multiple cloning site (MCS) of a pCDNA3-internal ribosomal entry site (IRES)-GFP-vector that contained the cytomegalovirus (CMV) promoter, the MCS, the IRES, plus the green fluoresecent protein (GFP) coding sequence.
- 48 h after the first siRNA transfection, exchange the medium (3.9 ml) for a second time and transform the cells with either empty vector or SEC61A1 expression plasmid using Fugene HD according to the manufacturer’s protocol (final ratio of vector to Fugene HD is 4 μg vector to 16 μl Fugene HD). Briefly, prepare plasmids freshly in a separate microcentrifuge tube prior to the transformation procedure: Add 16 μl Fugene HD transformation reagent to 4 μg of each plasmid that is dissolved in 80 μl of OptiMEM. Gently vortex this mix and incubate at room temperature for 10 min. Add the plasmid mix (0.1 ml) dropwise to the seeded 5.2 x 105 HeLa cells (3.9 ml).
- After 48 h of plasmid expression, subject culture dishes to fluorescence microscopy prior to calcium imaging and harvesting. If necessary replace DMEM by PBS for better fluorescence signals. Record fluorescence images on a fluorescence microscope equipped with cooled CCD camera. Transformation efficiency can be determined by dividing the number of cells displaying GFP fluorescence by cells counted in the brightfield mode and should be above 80%. A typical result is shown in Fig. 3.
4. Live cell calcium imaging
- Prior to measurement, transfer the cover slip coated with transfected HeLa cells in a separate 3.5 cm culture dish. Load the cells with 4 μM FURA-2 AM in 1 ml DMEM for 45 min at 25°C in the dark11,12.
- Fix a 25 mm cover slip in the iMIC metal chamber and wash cells twice and incubate in a calcium-free buffer (each time 300 μl).
- Start collecting data on an iMIC microscope with polychrome V by alternately exciting at 340 nm and 380 nm and measuring the emitted fluorescence at 510 nm (Filter set: beamsplitter, 565 nm; emitter, 605/70 nm). Sample images containing 20-50 cells/frame every 3 s. Record FURA-2 signals as the ratios F340/F380, where F340 and F380 correspond to the background-subtracted fluorescence intensity at 340 and 380 nm, respectively.
- After 1 min, treat cells with puromycin (500 μM) in calcium-free buffer or with the same buffer. Alternatively, treat cells with a small molecule inhibitor (such as 10 μM trifluoperazine or 100 μM ophibolin A) or with the calcium-free buffer.
- After ratiometric measurements are carried out for 2 or 10 min, add thapsigargin (1 μM) and continue the measurements.
- Eventually, cytoslic [Ca2+] is estimated from ratio measurements by an established calibration method.2 Analyze data by Excel 2007 and Origin 6.1.
5. Representative Results:
So far, we addressed the question of whether silencing of the SEC61A1 gene affects calcium (Ca2+) leakage from the ER (Fig. 1). The SEC61A1 gene was silenced by two different siRNAs in HeLa cells for 96 hours. While silencing hardly affected cell growth and viability, protein transport into the ER of semi-permeabilized cells was almost completely inhibited. Furthermore, the SEC61A1 silenced cells were severely affected with respect to Ca2+ leakage from the ER. The effect of gene silencing was reversed by expression of the SEC61A1 gene. Thus, Sec61 complexes that are present in the ER membrane of all nucleated cells form Ca2+ leak channels that can be expected to play a crucial role in Ca2+ homeostasis. However, the presence of large, water-filled pores with uncontrolled ion permeability, as formed by Sec61 complexes in the ER membrane, would seriously interfere with the regulated release of Ca2+ from the ER lumen into the cytosol, an essential mechanism for intracellular signalling. We identified a calmodulin (CaM) binding motif in the cytosolic N-terminus of mammalian Sec61α that bound CaM but not Ca2+-free apocalmodulin with nanomolar affinity and sequence specificity. At the cellular level, two different CaM antagonists stimulated Ca2+ release from the ER in the presence but not in the absence of Sec61 channels (Figs 4 and 5). The latter was not observed when Sec61 channels were present that contained mutations in the IQ motif of Sec61α. Thus, Ca2+-CaM is involved in limiting Ca2+ leakage from the ER (Fig. 6).
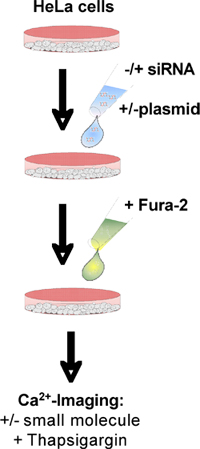
Figure 1. Flow chart. See text for details.
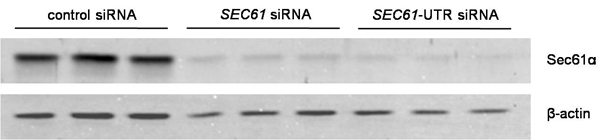
Figure 2. Data analysis for silencing efficiency. Silencing was evaluated by Western-blot analysis using antibodies that were directed against Sec61α and β-actin (loading control). The primary antibodies were visualized by using ECL Plex secondary antibodies and fluorescence imaging.
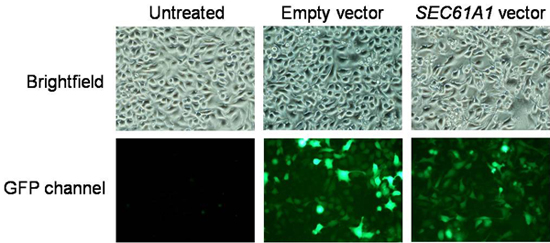
Figure 3. Data analysis for transfection efficiency. Images were recorded on a fluorescence microscope equipped with cooled CCD camera. Transformation efficiency can be determined by dividing the number of cells displaying GFP fluorescence by cells counted in the brightfield mode.
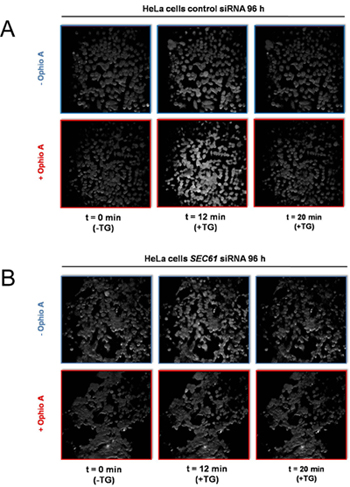
Figure 4. Screen shots from live cell calcium imaging of HeLa cells in the presence of control- or SEC61A1-siRNA and the presence or absence of the CaM-antagonist ophiobolin A. HeLa cells were treated with siRNA directed against SEC61A1 (B) or a negative control siRNA (A) for 96 h as indicated. These cells were loaded with the calcium indicator FURA-2 AM and incubated in a Ca2+ free buffer containing 0.5 mM EGTA, then buffer or ophiobolin A (Ophio A) in buffer was added and the incubation resumed. After 10 min, Ca2+ release was initiated by applying thapsigargin (TG) in the absence of external Ca2+ and the incubation was continued. Screen shots from the continuous calcium imaging were taken at the indicated times.
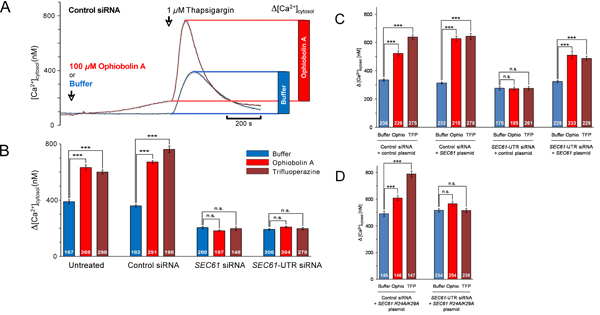
Figure 5. Data analysis for live cell calcium imaging experiments. (A) Kinetic and quantitative analysis of a series of experiments as depicted in Fig. 4A. Cytoslic [Ca2+] was estimated from ratio measurements by an established calibration method2. The effect of thapsigargin is shown as bar diagram. (B-D) Cells were treated with control siRNA or the indicated SEC61A1-siRNA for 48 h and then transformed with either control vector (C), or SEC61A1 expression plasmid (C), or mutant SEC61A1 expression plasmid (D) as indicated. After 48 h, calcium imaging experiments were carried out as in Figs 4 and 5A. Statistical analysis of the changes in the cytosolic Ca2+ concentration after the addition of thapsigargin in experiments such as presented in A are shown. P values < 0.001 were defined as significant by unpaired t test and are indicated by three asterisks (***), n.s., not significant. The numbers of cells that were analyzed are indicated. Average values are given, error bars represent standard errors of the means (s.e.m.). We note that these examples were adapted from ref. 13.
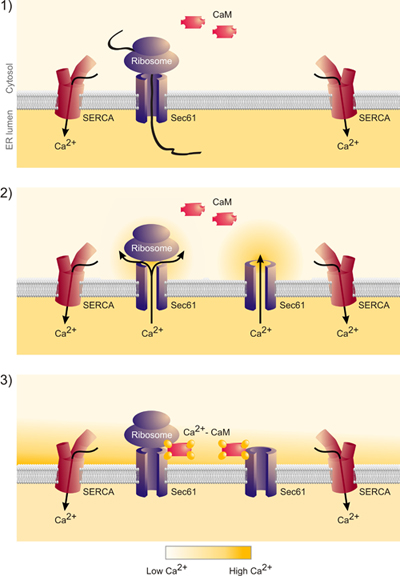
Figure 6. These data indicate that the release of nascent chains from the Sec61 complex indeed leads to calcium release from the ER and the formation of a calcium nanodomain around the cytosolic mouth of the Sec61 complex. This calcium is bound by calmodulin and calcium-calmodulin closes the Sec61 complex.”
Dyskusje
In mammalian cells, the endoplasmic reticulum (ER) plays a key role in protein biogenesis as well as in calcium signalling. Here, we have described an experimental system that allows us to directly address the role of one potential Ca2+ leak channel and to characterize its putative regulatory mechanisms13. This experimental system gives us the unique opportunities to i) evaluate the contribution of different ER membrane proteins to passive Ca2+ efflux from the ER in various cell types, ii) characterize the proteins and mechanisms that limit this passive Ca2+ efflux, and iii) study the effects of disease linked mutations in the relevant components.
We note that only viable cells should be analyzed and that overall viability should be above 80%. Therefore, cell viability is routinely evaluated employing Nuclear-ID blue/green cell viability reagent according to the manufacturer’s protocol. Furthermore, statistical analysis of the changes in the cytosolic Ca2+ concentration is mandatory. Therefore, the experiments have to be carried out for four different batches of cells and two coverslips with at least 20 cells have to be analyzed for each condition in a single experiment. We note that the various experiments have to be carried out at the same time after seeding on the cover slips.
Ujawnienia
No conflicts of interest declared.
Podziękowania
S.L. was supported by a fellowship from the DFG (Graduate Research School 845). This work was supported by grants from the DFG (SFB 530/C1 & FOR 967) and by HOMFOR.
Materiały
| Name | Company | Catalog Number | Comments |
| DMEM+GlutaMAX | Invitrogen | 31966 | |
| OptiMEM+GlutaMAX | Invitrogen | 51985 | |
| FBS | Biochrom AG | S0115 | |
| Penicillin/ Streptomycin | PAA Laboratories | P11-010 | |
| HiPerFect | Qiagen | 301707 | |
| Fugene HD | Roche Group | 04709713 | |
| Nuclear-ID Blue/Green cell viability reagent | Enzo Life Sciences | ENZ-53004 | |
| FURA-2 AM | Invitrogen | F-1221 | |
| Puromycin | Sigma-Aldrich | P 7255 | |
| Thapsigargin | Invitrogen | T-7459 | |
| Ophiobolin A | Enzo Life Sciences | ALX-270-109 | |
| Trifluoperazine | Sigma-Aldrich | T 6062 | |
| Countess® Automated Cell Counter | Invitrogen | ||
| Typhoon-Trio imaging system | GE Healthcare | ||
| TE2000-S microscopewith DS-5Mc camera | Nikon Instruments | ||
| iMIC microscope with polychrome V | Till Photonics |
Odniesienia
- Clapham, D. E. Calcium signalling. Cell. 131, 1047-1058 (2007).
- Lomax, R. B., Camello, C., Van Coppenolle, F., Petersen, O. H., Tepikin, A. V. Basal and physiological Ca2+ leak from the endoplasmic reticulum of pancreatic acinar cells. J. Biol. Chem. 277, 26479-26485 (2002).
- Van Coppenolle, F. Ribosome-translocon complex mediates calcium leakage from endoplasmic reticulum stores. J. Cell Sci. 117, 4135-4142 (2004).
- Flourakis, M. Passive calcium leak via translocon is a first step for iPLA2-pathway regulated store operated channel activation. FASEB J. 20, 1215-1217 (2006).
- Giunti, R., Gamberucci, A., Fulceri, R., Banhegyi, G. Both translocon and a cation channel are involved in the passive Ca2+ leak from the endoplasmic reticulum: a mechanistic study on rat liver microsomes. Arch. Biochem. Biophys. 462, 115-121 (2007).
- Ong, H. L., Liu, X., Sharma, A., Hegde, R. S., Ambudkar, I. S. Intracellular Ca2+ release via the ER translocon activates store operated calcium entry. Pflugers Arch. Eur. J. Physiol. 453, 797-808 (2007).
- Amer, M. S. Translocon closure to Ca2+ leak in proliferating vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 296, 910-916 (2009).
- Tu, H. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 126, 981-993 (2006).
- Hamman, B. D., Hendershot, L. M., Johnson, A. E. BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell. 92, 747-758 (1998).
- Alder, N. N., Shen, Y., Brodsky, J. L., Hendershot, L. M., Johnson, A. E. The molecular mechanism underlying BiP-mediated gating of the Sec61 translocon of the endoplasmic reticulum. J. Cell Biol. 168, 389-399 (2005).
- Aneiros, E., Philipp, S. E., Lis, A., Freichel, M., Cavalie, A. Modulation of Ca2+ signalling by Na+/Ca2+ exchangers in mast cells. J. Immunol. 174, 119-130 (2005).
- Gross, S. A. TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J. Biol. Chem. 284, 34423-34432 (2009).
- Erdmann, F. Interaction of calmodulin with Sec61a limit Ca2+ leakage from the endoplasmic reticulum. EMBO J. 30, 17-31 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone