Method Article
Mouse Oocyte Microinjection, Maturation and Ploidy Assessment
W tym Artykule
Podsumowanie
Oocytes are prone to aneuploidy due to errors in chromosome segregation during meiotic maturation. Aneuploid eggs can cause infertility, miscarriages or developmental disorders like Down syndrome. Here, we describe methods to introduce materials of choice into oocytes and methods to study meiotic maturation and assess ploidy.
Streszczenie
Mistakes in chromosome segregation lead to aneuploid cells. In somatic cells, aneuploidy is associated with cancer but in gametes, aneuploidy leads to infertility, miscarriages or developmental disorders like Down syndrome. Haploid gametes form through species-specific developmental programs that are coupled to meiosis. The first meiotic division (MI) is unique to meiosis because sister chromatids remain attached while homologous chromosomes are segregated. For reasons not fully understood, this reductional division is prone to errors and is more commonly the source of aneuploidy than errors in meiosis II (MII) or than errors in male meiosis 1,2.
In mammals, oocytes arrest at prophase of MI with a large, intact germinal vesicle (GV; nucleus) and only resume meiosis when they receive ovulatory cues. Once meiosis resumes, oocytes complete MI and undergo an asymmetric cell division, arresting again at metaphase of MII. Eggs will not complete MII until they are fertilized by sperm. Oocytes also can undergo meiotic maturation using established in vitro culture conditions 3. Because generation of transgenic and gene-targeted mouse mutants is costly and can take long periods of time, manipulation of female gametes in vitro is a more economical and time-saving strategy.
Here, we describe methods to isolate prophase-arrested oocytes from mice and for microinjection. Any material of choice may be introduced into the oocyte, but because meiotically-competent oocytes are transcriptionally silent 4,5 cRNA, and not DNA, must be injected for ectopic expression studies. To assess ploidy, we describe our conditions for in vitro maturation of oocytes to MII eggs. Historically, chromosome-spreading techniques are used for counting chromosome number 6. This method is technically challenging and is limited to only identifying hyperploidies. Here, we describe a method to determine hypo-and hyperploidies using intact eggs 7-8. This method uses monastrol, a kinesin-5 inhibitor, that collapses the bipolar spindle into a monopolar spindle 9 thus separating chromosomes such that individual kinetochores can readily be detected and counted by using an anti-CREST autoimmune serum. Because this method is performed in intact eggs, chromosomes are not lost due to operator error.
Protokół
1. Mouse oocyte collection
- To maximize the number of antral follicles isolated from each mouse, intraperitoneally inject sexually mature female mice (we use 6-week-old CF-1 mice from Harlan) with 5 IU of pregnant mare's serum gonadotropin (PMSG).
- Prepare the collection medium (MEM/PVP) (3 ml/mouse) by adding milrinone to 2.5 μM and warm it at 37°C. Milrinone is a phosphodiesterase inhibitor that maintains meiotic arrest once oocytes are removed from follicles. Alternatively, 3-isobutyl-1-methylxanthine (IBMX) (0.2 mM) or dibutyryl-cyclic adenosine monophosphate (dbcAMP) (100 μg/ml) can be used. Prepare the culture medium by adding glutamine (1 mM) and milrinone (2.5 μM) to 1 ml of CZB and allow it to equilibrate in the incubator for at least an hour. Set up the microdrops of each in a Petri dish and overlay with mineral oil. The CZB dish is placed into the incubator while the MEM/PVP dish remains outside on a slide warmer. Other collection and culture medium that are commercially available (M2 and M16 from Specialty media or Sigma) are also routinely used.
- Approximately 48 h after PMSG priming, sacrifice the mouse using CO2 sedation followed by cervical dislocation, dissect the ovaries and place them into a watchglass containing pre-warmed MEM/PVP + milrinone (MEM/PVP+M).
- Using a 1 ml insulin syringe, anchor the ovaries to the dish and release the antral follicles by puncturing them several times with 27-gauge (or sewing) needles that are fastened together.
- While looking through a dissection microscope, collect cumulus-oocyte complexes using a mouth-operated glass pipette. It is helpful to have a microscope with a lot of contrast. Alternatively, the medium containing the tissue and cells can be pipetted on to a lid of a plastic Petri dish. Only collect large, antral follicles and not smaller pre-antral follicles or denuded oocytes. Once collected, transfer the complexes to a microdrop of MEM/PVP+M that is on the slide warmer and under oil.
- Using a small pipette (slightly larger than the diameter of the oocyte), pipette up and down the complexes to detach the cumulus cells. Transfer the denuded oocytes with a larger pipette into a microdrop of CZB + milrinone (CZB+M) and place in the incubator.
- Allow oocytes to recover for at least 1 h in the incubator prior to manipulation.
2. Oocyte microinjection
- Make injection pipettes by pulling borosilicate-glass capillary tubing in a mechanical puller. We use a Flaming-Brown micropipette puller (Model P-97) with the following settings: P=500, Heat=300, Pull=150, Vel=100, Time=150.
- Prepare the microinjection platform by placing a 5 μl drop of MEM/PVP+M as close as possible to a 0.5 μl drop of injection material of choice (e.g. siRNA, cRNA, morpholino oligonucleotide, etc.) on a 1-well chamber slide that has the media chamber removed. Cover with mineral oil and place on the microscope stage (Fig. 1).
- Place injection and holding pipettes into the holders and position into the drop of MEM/PVP+M. Turn on the nitrogen tanks that are connected to the picoinjector and anti-vibration table. Open the tip of the injection pipette by gently tapping it against the holding pipette while filling it with medium. If the opening is too large, the medium will move in and out quickly and the needle will kill the cell. Pipettes with too small of an opening often clog easily.
- Transfer oocytes (5-10) from the incubator to the MEM/PVP+M drop on the platform. Before injecting, setup the picoinjector. We use the following parameters on our picoinjector (Model PLI-100 from Harvard Apparatus): PBal=2.5-3.5 psi, PInj=7.8 psi, PClear= 10-12 psi, time= 3s. This results in an injection volume of 5-10 pL.
- While pressing CLEAR, move the injection needle to the material drop and fill. Return it to the medium drop.
- Use the holding pipette to capture an oocyte and align the injection needle, oocyte and holding pipette along the x-axis.
- Under higher magnification, advance the injection pipette through the oocyte being careful to avoid the nucleus. Once the plasma membrane is pierced, press INJECT. After injection, withdraw the needle. Release the oocyte and repeat. Once all the oocytes are injected, return them to the incubator. Hold in CZB+M for desired amount of time; this time is dependant upon the experimental aim (i.e. siRNA and morpholino knockdown (up to 24 h), overexpression (3-12 h)). Please note that incubations longer than 24 h will compromise meiotic maturation.
3. Oocyte maturation
- After incubation wash the oocytes through several drops of CZB (maturation medium) and transfer to a microdrop of maturation medium under oil and place in the incubator.
- Allow 16 h for complete maturation to metaphase of meiosis II.
4. Ploidy analysis
- Prepare CZB + monastrol (100 μM) and place 750 μl into the well of an organ culture dish that has water in the outer ring.
- Wash eggs through several drops of CZB + monastrol and transfer them into the organ culture dish. Place in the incubator for 1 h.
- Fix the eggs by transferring them into a watchglass containing 2% paraformaldehyde in PBS for 20 minutes at room temperature. Transfer into another watchglass with blocking solution. This dish can be stored at 4° C until processed.
- Transfer to permeabilization solution (PBS + 0.3% BSA + 0.1% TritonX-100 + 0.02% NaN3) in a watchglass for 15 minutes at room temperature. Rinse through several large volumes of blocking solution (PBS + 0.3% BSA + 0.01 Tween-20 + 0.02% NaN3).
- The rest of the procedure is performed on the lid of a 96-well plate in a humidified chamber at room temperature that is protected from light. The drops (˜25 μl) are placed inside the circular indentations. Transfer eggs to blocking solution for 15 minutes.
- To label centromeres, transfer eggs to a drop of blocking solution containing CREST anti-serum at 1:40. Incubate for at least 1 h. Wash through 3 drops of blocking solution, incubating 15 minutes in each.
- Transfer eggs to a drop of blocking solution containing Cy5- or Alexa-fluor-594-conjugated anti-human IgG (1:200) and incubate for 1h. Repeat washing steps as above except for the last step, include Sytox Green (1:5,000) in the blocking solution to detect chromosomes. Mount in 5 μl of Vectashield. To prevent crushing of the eggs, put 4 small spots of petroleum jelly at the corners of where the coverslip will be. Place a coverslip on top and seal with nail polish. Store in a slide box at 4° C until processing via confocal microscopy.
- While imaging with a 100X objective, capture 0.4 μm steps in the Z-plane and image the entire region of the metaphase spindle. Count centromeres using imaging software such as Image J (NIH).
5. Representative Results:
Figure 2 is a Z-projection from a euploid egg. At metaphase of MII, euploid mouse eggs contain 20 pairs of chromosomes and therefore have 40 centromeres. Occasionally chromosomes fail to spread despite monastrol treatment. This situation makes it difficult to reliably count centromeres and we therefore do not include these eggs in our analyses. Occasionally, it may be challenging to determine whether a CREST-immunoreactive "spot" is 1 or 2 centromeres. Using programs such as Image J is useful because one can analyze each Z-section while carefully noting the orientation of the chromosomes, the number of sections in which a "spot" is detected and the pixel intensity the "spots" have. Depending where the meiotic spindle is positioned relative to the polar body, the regions of DNA can overlap and these samples should not be included in the analysis.
Unmanipulated, in vivo ovulated eggs from reproductively young mice have low rates of aneuploidy (˜1-2%). However, for reasons not understood, microinjection and in vitro maturation procedures can increase this rate upwards of 10%. Therefore, it is critical that control injected oocytes are included in any microinjection study.
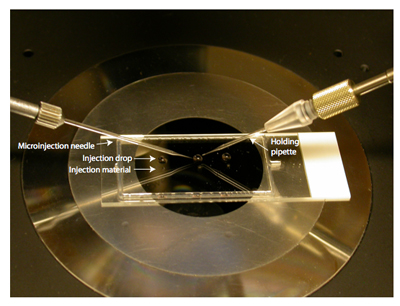
Figure 1. Microinjection dish set up. A chamber slide with 5 μl drops of MEM/PVP + M just above 0.5μl drops of injection solution. Cover with mineral oil. In this example there are 3 drops for 3 different injection solutions and the slide is sitting on the stage of a microscope. To the left is the microinjection needle and to the right is the holding pipette. Note that there is a reflection of the needle holders.
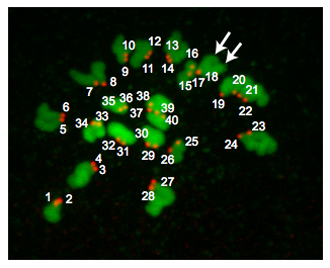
Figure 2. Ploidy results. A Z-projection of a euploid metaphase II egg. DNA is colored in green and kinetochores are colored in red. The arrows point to 2 distinct chromatid arms indicating that kinetochores (#17 and #18) overlap. Euploid mouse eggs contain 20 kinetochore pairs (40 total "spots"). An aneuploid egg would contain any variation on this number. If this procedure were conducted on metaphase MI oocytes, there would be 40 kinetochore pairs (80 total "spots").
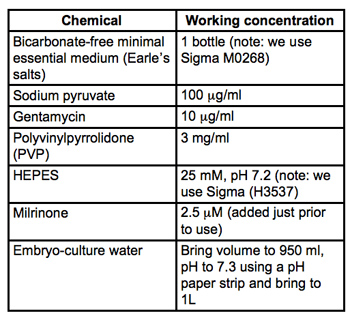
Table 1. Recipe for collection and microinjection medium (MEM/PVP + M). All materials are embryo-culture grade and from Sigma-Aldrich. Filter sterilize through 0.22μm PVDF filters (we use Milipore Stericups) and store at 4°C.
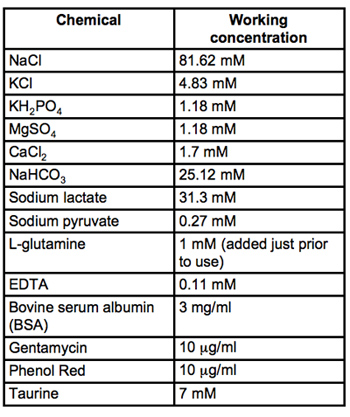
Table 2. Recipe for CZB medium. All materials are embryo-culture grade and from Sigma-Aldrich. Filter sterilize through 0.22μm PVDF filters (we use Milipore Stericups) and store at 4°C.
Dyskusje
Microinjection of oocytes is a powerful method to study mechanisms that regulate meiotic maturation 10,11, 12, 13. This method provides an economical way to test hypotheses prior to making a large investment in developing transgenic and targeted mouse models. Oocyte collection and microinjection techniques require more time to master than typical cell biology procedures. Specific obstacles with collection often include controlling the mouth pipette, pulling the proper size glass pipette for collection and stripping of the somatic cells and increasing collection speed to minimize the time in which oocytes are outside of the incubator. We recommend practicing many times prior to doing experiments. Transferring oocytes between microdrops while maintaining the same number of cells is a great way to become comfortable with this method.
Cell death commonly occurs while learning microinjection. This could occur for a number of reasons, including injection of too large of a volume of material (i.e. the injection needle opening is too big), hitting the nucleus with the injection needle, piercing the opposite side of the oocyte or that the material injected is toxic to the oocyte. Practicing with injecting buffer into oocytes until your survival rate is at least 50% is key to mastering this technique. If oocytes fail to mature it is likely that the milrinone was not diluted enough. We recommend rinsing the oocytes through many large drops of milrinone-free CZB before maturation.
The ploidy analysis following microinjection is one of many assays to assess meiotic maturation. Other routine analyses we use in the laboratory include monitoring the kinetics by which the oocytes progress through meiosis, immunofluorescence to analyze spindle formation and chromosome alignment and egg activation or in vitro fertilization to assess the developmental consequences of the oocyte manipulation 14,15, 16, 17.
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was conducted in Richard M. Schultz's laboratory. The authors would also like to acknowledge Michael Lampson for conceptualizing the centromere-counting assay and access to his confocal microscope. Teresa Chiang and Francesca Duncan assisted in optimizing the centromere-counting assay. Paula Stein is supported by HD022681 (to RMS) and Karen Schindler is supported by HD055822.
Materiały
| Name | Company | Catalog Number | Comments |
| Table of specific reagents: | |||
| Milrinone | Sigma-Aldrich | M4659 | Resuspend in DMSO at 2.5mM |
| Mineral oil | Sigma-Aldrich | M5310 | Only use embryo-tested, sterile-filtered |
| CREST autoserum | Immunovision | HCT-0100 | |
| Sytox Green | Invitrogen | 57020 | |
| Anti-human Alexa 594 | Invitrogen | A-11014 | |
| Vectashield | Vector Laboratories | H-100 | |
| Paraformaldehyde | Polysciences, Inc. | 577773 | |
| Albumin from bovine serum | Sigma-Aldrich | A3294 | |
| PMSG | Calbiochem | 367222 | |
| Monastrol | Sigma-Aldrich | M8515 | Resuspend in DMSO at 100mM |
| Tween-20 | Sigma-Aldrich | 274348 | |
| TritonX-100 | Sigma-Aldrich | X-100 | |
| Table of specific equipment: | |||
| Mouthpiece | Biodiseno | MP-001-Y | |
| Watchglass | Electron Microscopy Sciences | 70543-30 | |
| Syringe | BD Biosciences | 309623 | 1ml, 27G1/2 |
| 60 mm Petri Dish | Falcon BD | 351007 | |
| Glass Pasteur pipets | Fisher Scientific | 13-678-200 | |
| Side warmer | Fisher Scientific | Any standard model | |
| Dissection microscope | Any standard model | ||
| Chamber Slide | Nalge Nunc international | 177372 | |
| Capillary Tubing | Drummond Scientific | 1-000-0500 | microcaps |
| Pipette Puller | Flaming-Brown micropipette puller | Model P-97 | |
| Inverted microscope | Nikon Instruments | Any standard model | |
| Micromanipulators | Eppendorf | Any standard model | |
| Picoinjector | Harvard Apparatus | Model PLI-100 | Any standard model |
| CO2 tanks | For incubator | ||
| N2 tank | For table and injector | ||
| Anti-vibration table | Technical Manufacturing Corp. | Any standard model | |
| Incubator | Any standard model | ||
| Holding pipettes | Eppendorf | 930001015 | Vacutip |
| Confocal microscope | Leica Microsystems | Any standard model | |
| Dissection tools | Fine Science Tools | Any standard model | |
| Humidified chamber | We use tupperware | ||
| Lid of 96 well plate | Nalge Nunc international | 263339 | |
| Microscope slides | Fisher Scientific | 12-544-3 | |
| Coverslips | Thomas Scientific | 6663-F10 | Thickness will vary for particular microscopes |
| Center well organ culture dish | Fisher Scientific | 353037 | 60 X 15mm |
Odniesienia
- Hunt, P. A., Hassold, T. J. . Science. 296 (5576), 2181-2181 (2002).
- Hassold, T., Hall, H., Hunt, P. . Hum Mol Genet. 16 Spec No. 2, R203-R203 (2007).
- Chatot, C. L., Ziomek, C. A., Bavister, B. D. . Journal of reproduction and fertility. 86 (2), 679-679 (1989).
- Schultz, M. R. . Oogenesis and the control of meiotic maturation. , (1986).
- Moore, G. P., Lintern-Moore, S. . Biol Reprod. 18 (5), 865-865 (1978).
- Hodges, C. A., Hunt, P. A. . Chromosoma. 111 (3), 165-165 (2002).
- Schindler, K., Schultz, R. M. . Cell Cycle. 8 (7), 1090-1090 (2009).
- Chiang, T., Duncan, F. E., Schindler, K. . 20 (17), 1522-1522 (2010).
- Mayer, T. U., Kapoor, T. M., Haggarty, S. J. . Science. 286 (5441), 971-971 (1999).
- Stein, P. . Cold Spring Harbor protocols. 2009 (1), pdb prot5135-pdb prot5135 (2009).
- Stein, P. . Cold Spring Harbor protocols. 2009 (1), pdb prot5132-pdb prot5132 (2009).
- Reis, A., Chang, H. Y., Levasseur, M. . Nat Cell Biol. 8 (5), 539-539 (2006).
- Schuh, M., Ellenberg, J. . Cell. 130 (4), 484-484 (2007).
- Backs, J., Stein, P., Backs, T. . Proceedings of the National Academy of Sciences of the United States of America. 107 (1), 81-81 (2010).
- Schindler, K., Schultz, R. M. . Biol Reprod. 80 (4), 795-795 (2009).
- Kudo, N. R., Wassmann, K., Anger, M. . Cell. 126 (1), 135-135 (2006).
- Shoji, S., Yoshida, N., Amanai, M. . The EMBO Journal. 25 (4), 834-834 (2006).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone