Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Biophysical Assays to Probe the Mechanical Properties of the Interphase Cell Nucleus: Substrate Strain Application and Microneedle Manipulation
W tym Artykule
Podsumowanie
We present two independent, microscope-based tools to measure the induced nuclear and cytoskeletal deformations in single, living adherent cells in response to global or localized strain application. These techniques are used to determine nuclear stiffness (i.e., deformability) and to probe intracellular force transmission between the nucleus and the cytoskeleton.
Streszczenie
In most eukaryotic cells, the nucleus is the largest organelle and is typically 2 to 10 times stiffer than the surrounding cytoskeleton; consequently, the physical properties of the nucleus contribute significantly to the overall biomechanical behavior of cells under physiological and pathological conditions. For example, in migrating neutrophils and invading cancer cells, nuclear stiffness can pose a major obstacle during extravasation or passage through narrow spaces within tissues.1 On the other hand, the nucleus of cells in mechanically active tissue such as muscle requires sufficient structural support to withstand repetitive mechanical stress. Importantly, the nucleus is tightly integrated into the cellular architecture; it is physically connected to the surrounding cytoskeleton, which is a critical requirement for the intracellular movement and positioning of the nucleus, for example, in polarized cells, synaptic nuclei at neuromuscular junctions, or in migrating cells.2 Not surprisingly, mutations in nuclear envelope proteins such as lamins and nesprins, which play a critical role in determining nuclear stiffness and nucleo-cytoskeletal coupling, have been shown recently to result in a number of human diseases, including Emery-Dreifuss muscular dystrophy, limb-girdle muscular dystrophy, and dilated cardiomyopathy.3 To investigate the biophysical function of diverse nuclear envelope proteins and the effect of specific mutations, we have developed experimental methods to study the physical properties of the nucleus in single, living cells subjected to global or localized mechanical perturbation. Measuring induced nuclear deformations in response to precisely applied substrate strain application yields important information on the deformability of the nucleus and allows quantitative comparison between different mutations or cell lines deficient for specific nuclear envelope proteins. Localized cytoskeletal strain application with a microneedle is used to complement this assay and can yield additional information on intracellular force transmission between the nucleus and the cytoskeleton. Studying nuclear mechanics in intact living cells preserves the normal intracellular architecture and avoids potential artifacts that can arise when working with isolated nuclei. Furthermore, substrate strain application presents a good model for the physiological stress experienced by cells in muscle or other tissues (e.g., vascular smooth muscle cells exposed to vessel strain). Lastly, while these tools have been developed primarily to study nuclear mechanics, they can also be applied to investigate the function of cytoskeletal proteins and mechanotransduction signaling.
Protokół
1. Substrate strain application
The measurement of normalized nuclear strain includes the preparation of strain dishes with transparent, elastic silicone membranes as cell culture surface, plating cells onto the dishes, and acquiring images of the cells before, during and after (uniaxial or biaxial) strain application.
Preparation of silicone membrane dishes and adherence of cells
- Each strain dish consists of a custom-made bottomless plastic dish with a diameter of 3" and a plastic O-ring to hold a silicone membrane, which serves as the cell culture substrate. For preparation of the strain dishes, clamp a 4" x 4" piece of silicone membrane between the O-ring and the dish. Carefully cut away the excess membrane, rinse with deionized water, and autoclave the strain dishes.
- Mark a reference point in the bottom center of the membrane (on the outside) before coating the silicone membranes with extracellular matrix molecules (e.g., fibronectin). This landmark will help identify the same cells during the strain experiments. (Optional: For uniaxial strain application, two parallel stripes of scotch tape are applied around the reference point to restrict deformation of the membrane in one dimension.)
- To provide optimal cell attachment, coat the silicone membranes with 3 μg/ml fibronectin diluted in 10 ml PBS or any suitable extracellular matrix proteins. Cover the strain dish with an inverted 10cm polystyrene dish, and incubate the dishes over night at 4°C.
- On the next day, rinse the membranes once with Phosphate Buffered Saline (PBS) to remove excess protein. Fill dish with 10ml of growth medium (Dulbecco's Modified Eagles Medium (high glucose) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin) and set aside.
- Mouse embryonic fibroblasts are trypsinized with 0.05% trypsin and seeded in growth medium at approximately 30% confluence onto the coated silicone membrane dish and incubate for 24 – 48 hours under normal culture conditions.
Substrate strain experiments
- Set-up the microscope for experiments. The experiments are performed on an inverted microscope with a digital camera suited for fluorescence microscopy, phase contrast or DIC, using a 60x objective and appropriate image acquisition software (e.g., IPLab or Metamorph). An upright microscope is not suitable for this application. The strain device consists of a base plate that fits onto the microscope stage and holds a central cylindrical platen that serves to apply strain to the central section of the silicone membrane, a movable plate that holds the strain dish and that can slide up and down on four guidance pins, as well as a 5-lb weight plate to apply a load.
- In order to visualize nuclei, incubate the cells in the strain dish with 1 μg/mL of Hoechst 33342 for 15 min at 37°C. Aspirate off the medium and replace with 15 ml phenol-red free growth medium (phenol-red free Dulbecco's Modified Eagles Medium (high glucose) with 25 mM Hepes, supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin). Screw the strain dish into the dish holder plate. Carefully apply grease (Braycote 804 Vacuum Grease) to the perimeter of the bottom of the silicone membrane to ensure gliding of the membrane along the central platen. Make sure to keep the central section of the membrane clear.
- Place the base plate onto the microscope stage. Mount dish holder plate carefully on the base plate. Assure that in the initial resting position, the silicone membrane of the strain dish loosely rests on the central platen.
- First, focus on bottom of the silicone membrane and find the central black reference dot. The dot will serve as the starting point for all image acquisitions and aids in locating the same cells during and after stretch. We use a custom-written automated imaging program to store the positions of cells and to relocate these cells during the experiment, but this can also be achieved manually.
- Starting from the dot, adjust the focus to visualize the cells and the top of the silicone membrane. Locate well-spread cells with centrally located nuclei and acquire a phase contrast and a fluorescence image of the nuclear Hoechst stain. The phase contrast image should focus on the cell outline and the silicone membrane, while the fluorescence images should focus on the central plane of the nucleus.
- After acquiring images of 5 to 15 cells, move back to the central dot. Slowly apply the weight to the strain dish, resulting in uniform strain application in the center of the dish. The maximal applied substrate strain is limited by nylon spacers placed on the vertical alignment pins (guidance pins).
- Focus on the bottom of the silicone membrane and locate the reference dot again. Starting from the dot, relocate the same cells and again acquire a phase contrast and a fluorescence image of the cells and nuclei under full strain, trying to closely match the focal planes of the initial images. This process should not exceed 10 min to avoid active remodeling and adaptation of the cell to the strained substrate.
- After all the corresponding images have been acquired, move the microscope stage back to the starting point. Carefully remove the weight from the dish holder plate and allow the silicone membrane to relax. If necessary, gently push up the strain dish until it is in the initial position. Then acquire phase contrast and fluorescence images of the post-strain cells as described above for the strain images.
Analysis
- Images of cells and fluorescently labeled nuclei before, during, and after strain application are analyzed to compute the normalized nuclear strain. In our laboratory, we use a custom-written MATLAB script for the analysis, but several alternative options are available. The analysis is performed in three steps.
- First, to calculate the applied substrate strain, the positions of 3 to 6 control points located on the membrane are manually matched between the corresponding pre-, full-, and post-strain images. The MATLAB program then computes the applied membrane strain by comparing the positions of matching control points between the pre-strain and full-strain images and also the residual strain between the pre-strain and the post-strain images. At the same time, the control points are used to register the image pairs, which will help to detect damaged or detaching cell (see Figure 1).
- In a second step, nuclei are manually selected using a separate MATLAB program that calculates nuclear strain for each individual nucleus by matching either nuclear size or intranuclear markers between corresponding pre-, full-, and post-strain fluorescent images. To account for small variations in applied membrane strain between different experiments, we express results as Normalized Nuclear Strain, defined as the ratio of induced nuclear strain to applied membrane strain that is computed for each nucleus. The MATLAB scripts are available from the Lammerding laboratory upon request.
- Finally, each nucleus is validated, excluding measurements from cells that detach or become damaged during strain application (Figure 1).
2. Microneedle manipulation assay
Preparation of dishes, adherent cells, and microneedles
- Incubate 35 mm glass bottom cell culture dishes with a low concentration of fibronectin (0.5ug/ml) in Hank's Buffered Salt Saline (HBSS) or any suitable extracellular matrix proteins for 2 hours at 37°C. Wash dishes with HBSS two times and add 2 ml of growth medium to the dish before proceeding to the next step.
- Mouse embryonic fibroblasts are trypsinized with 0.05% trypsin and seeded in 2 ml growth medium seeded at 7.5 x 104 cells/ml onto the fibronectin-coated glass bottom dishes. Place cells back in incubator overnight. Place cells back in incubator overnight. One should optimize the number of cells to obtain single, adherent, non-confluent cells for other cell types.
- Pull the microneedles, made of borosilicate capillaries, to tip diameters of approximately 1 to 3 μm with a commercial pipette puller (e.g., Sutter Instrument Company).
Microneedle manipulation experiment
- The next day, incubate the cells with MitoTracker mitochondrial stain (600 μM; Invitrogen) and Hoechst 33342 nuclear stain (1 μg/mL) added to growth medium for 30 minutes in a 37°C incubator.
- Wash the cells one time in HBSS for 5 minutes at room temperature and then add phenol-red free growth medium to the cells for imaging.
- Acquire one image of the single cell without the microneedle inserted into the cytoskeleton in phase contrast, one fluorescent image of the Hoechst 33342 stain and one fluorescent image of the mitochondrial stain, with a 60x objective (0.70 N.A., Plan-Achromat) on an inverted microscope with a digital charge-coupled device camera.
- Using a micromanipulator (e.g., InjectMan NI 2, Eppendorf), carefully insert the microneedle into the cytoplasm of a cell a fixed distance (typically 5 μm) away from the nuclear periphery and take one phase contrast image, one fluorescent image of the Hoechst 33342 stain and one fluorescent image of the mitochondrial stain. For this application, it helps to control the micromanipulator through a computer, e.g., Windows Hyperterminal, to achieve consistent micromanipulation procedures.
- Move the microneedle, a specific distance (typically 10 or 20 μm) towards the cell periphery at 1 μm/sec while simultaneously collecting fluorescence and phase contrast images every 10 seconds. As the microneedle is moving to a specific distance toward the cell periphery. With the chosen parameters, as the microneedle is moving to a specific distance toward the cell periphery, this will correspond to 2-3 frames during the manipulation process.
- Finally acquire additional images after the microneedle is removed from the cytoskeleton.
Analysis
- Displacement maps are computed using a custom-written MATLAB script based on tracking fluorescently labeled features of the nucleus and cytoplasm. (The MATLAB script is available from the Lammerding laboratory upon request). The program uses a normalized cross-correlation algorithm between small image regions (approximately 10 μm x 10 μm in size and spaced 5 μm apart) in subsequent image frames. For each region center, the displacement in the x- and y-directions are computed as the shift between the original location and the newly identified position and displayed as a displacement vector and also stored as numerical values. From the displacement maps, average displacements within predefined regions can be computed. Note that cytoskeletal displacements are based on the fluorescence channel for cytoskeletal markers (e.g., MitoTracker mitochondrial stain), while nuclear displacements are computed from the fluorescence channel corresponding to the Hoechst 33342 signal. For our application, we routinely examine the following regions: (i) cytoskeletal strain at the strain application site, i.e., the microneedle insertion site; (ii) nuclear strain in a region inside the nucleus towards the strain application site; (iii) nuclear strain in a nuclear region away from the application site; and (iv) cytoskeletal strain in a cytoplasmic region across the nucleus. In addition, one can also directly measure nuclear elongation from phase contrast or Hoechst 33342 fluorescence image sequences. In this case, applied nuclear strain is calculated by dividing the nuclear elongation (ΔL = L – L0) by the initial length, L0, where L is the final length of the nucleus at the end of strain application and L0 is the initial length of the nucleus. For cells with an intact nucleo-cytoskeletal coupling, the nucleus will elongate towards the strain application site. In contrast, in cells in which nucleo-cytoskeletal coupling is disrupted, i.e., forces are transmitted less efficiently between the cytoskeleton and the nucleus, the nucleus is expected to elongate significantly less in the direction of the strain application site. Thus, decreased nuclear deformations in response to cytoskeletal strain application imply a (partial) uncoupling between the nucleus and cytoskeleton.
3. Representative results:
Substrate strain application
We acquired images before, during, and after strain application to mouse embryonic fibroblasts from heterozygous and homozygous lamin A/C-deficient (Lmna+/– and Lmna–/–), and wild-type (Lmna+/+) mice and subsequently computed the normalized nuclear strain for each cell. After analysis, the nuclei are validated and cells that become damaged or retract during strain application are excluded from the analysis. Figure 1A depicts nuclei of three cells that are valid, whereas Figure 1B depicts cells that should be excluded from analysis. Normalized nuclear strain data are pooled from at least three independent experiments (each containing measurements from ~5–10 nuclei) and compared with other cell or treatment groups by statistical analysis. Increased normalized nuclear strain indicates reduced nuclear stiffness, as seen in cells with reduced expression of the nuclear envelope proteins lamin A/C (Figure 2).
Microneedle manipulation assay
For the microneedle manipulation assay, we imaged nuclear and cytoskeletal displacements during localized cytoskeletal strain application. Cells that become damaged or detached are excluded from the analysis. For the analysis, we measure the magnitude of the nuclear and cytoskeletal movements towards the force application site in single, adherent cells. For example, in Figure 3, we track mitochondrial (marker for the cytoskeleton) displacements before and after cytoskeletal strain and then plot the displacements as vectors. Each vector represents the displacement computed as the shift between the original location and the newly identified position. Regions with low image intensity or insufficient texture (e.g., regions outside the cell) are excluded from the analysis. The cytoskeletal and nuclear displacements are then quantified in select areas at increasing distances from the strain application site (Figure 4, areas corresponding to the colored boxes in inset). In mouse embryonic fibroblasts with intact nucleo-cytoskeletal coupling, forces are transmitted through the entire cells, resulting in induced nuclear and cytoskeletal deformations that slowly dissipate away from the strain application site (Figure 4). In contrast, fibroblasts with disturbed nucleo-cytoskeletal coupling (or altered cytoskeletal organization) display localized displacements near the application site, as shown in Figure 4 and only little induced deformations further away. Comparable cytoskeletal strain application at the microneedle insertion site (orange box) is observed for both control fibroblasts (mCherry alone) and fibroblasts with a disrupted nucleo-cytoskeletal coupling (DN KASH). However, induced nuclear and cytoskeletal displacements (blue, yellow, and red boxes) at other regions were significantly smaller in the fibroblasts with disrupted nucleo-cytoskeleton coupling (DN KASH) than in control cells (mCherry alone) (Figure 4). Thus, decrease in cytoskeletal and nuclear displacements away from the strain application site, indicates that force transmission between the cytoskeleton and nucleus was disturbed.
Importantly, we have also validated that mitochondria are suitable cytoskeletal marker, by conducting microneedle manipulation on mouse embryonic fibroblasts transfected with GFP- or mCherry actin and GFP-vimentin and fluorescently labeled with Mitotracker Green or Red. Cytoskeletal displacement maps were calculated independently from the fluorescent signal of the mitochondria and the actin or vimentin cytoskeleton. The average absolute displacement was computed for four distinct cytoskeletal regions at increasing distances away from the strain application site. The slope and R-squared values were computed from the linear regression between the measurements obtained from mitochondria and from actin or vimentin, respectively. For actin, the slope was 0.99 and the R2 value was 0.986; for vimentin, the slope was 1.04 and the R2 value was 0.971, confirming that mitochondrial displacements serve as reliable indicators for cytoskeletal deformations.
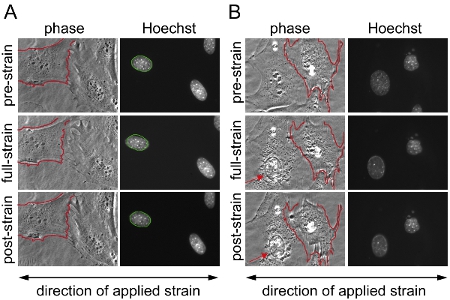
Figure 1. Substrate strain application on mouse embryonic fibroblasts (MEFs). Mouse embryonic fibroblasts spread over two distinct areas on the silicon membrane were imaged with phase contrast and fluorescence microscopy before, during and after application of 20% uniaxial strain. (A) Example of a successful experiment with valid nuclei from cells that survived the strain application without any damage or detachment and (B) example of cells that retract/partially detached during strain application; results from the cells depicted in (B) are excluded from the analysis. In (B), the cell on the left side shows signs of cytoskeletal damage and nuclear collapse (arrow), while the cell on the right side detaches partly and retracts during strain application. This can be an indication of excessive strain application. For better comparison, in (A) and (B) the border of one of the unstretched cell membranes is outlined in red and superimposed on the same cell during and after strain application. In (A) the border of the unstretched nucleus is outlined in green and superimposed on the same nucleus during and after strain application.
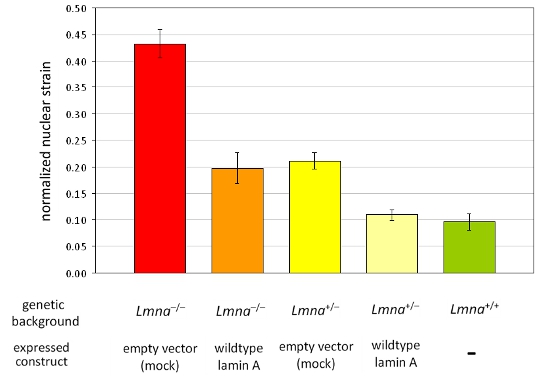
Figure 2. Analysis of normalized nuclear strain in a panel of different MEF cell lines. MEFs of the Lmna–/– and Lmna+/– genetic background ectopically expressing either an empty vector or wild-type lamin A were analyzed. In comparison to MEFs from wild-type littermates (Lmna+/+), loss of lamin A/C expression results in decreased nuclear stiffness that can be fully restored by reintroduction of wild-type lamin A. Notably, reduced nuclear stiffness is reflected by increased values of normalized nuclear strain. The error bars represent standard errors.

Figure 3. Microneedle manipulation assay to measure intracellular force transmission. Phase contrast (A, B) and fluorescence (C, D) images of a fibroblast labeled with nuclear stain (blue) and MitoTracker mitochondrial stain (green). A microneedle was inserted into the cytoskeleton at a defined distance from the nucleus (A and C) and subsequently moved towards the cell periphery (B, D). Cytoskeletal and nuclear displacements were quantified by tracking fluorescently labeled nucleus and mitochondria using a custom-written cross-correlation algorithm. (E) Displacement map of the final cytoskeletal (green) deformations computed from fluorescence image series; arrow length is magnified by 2x for better visibility. Scale bars, 10 μm.
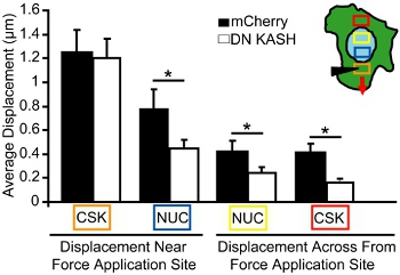
Figure 4. Analysis of intracellular force transmission during microneedle manipulation. Induced cytoskeletal and nuclear displacements during microneedle manipulation, measured in the areas corresponding to the colored boxes (inset in A). The orange box is the strain application site. Despite similar strain application in the cytoskeleton (orange box), induced nuclear and cytoskeletal displacements (blue, yellow, and red boxes) were significantly smaller in the mouse embryonic fibroblasts that with a disrupted nucleo-cytoskeletal coupling (DN KASH) compared to control (mCherry alone) cells.
Dyskusje
Substrate strain assay
Strain application has been successfully used by us and other groups to study induced nuclear deformations in cells subjected to mechanical stress and to investigate the contribution of specific nuclear envelope proteins to nuclear stiffness.4-8 The advantage of this technique is that it probes mechanical properties of living nuclei in their normal cellular and cytoskeletal environment and that the substrate strain application resembles physiological load applica...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported by the National Institutes of Health (R01 HL082792 and R01 NS059348) and the Brigham and Women's Hospital Cardiovascular Leadership Group Award.
Materiały
| Name | Company | Catalog Number | Comments |
| Fibronectin | EMD Millipore | FC010 | |
| MitoTracker Red FM and Green FM | Invitrogen | M22425 and M-7514 | |
| H–chst 33342 | Invitrogen | H3570 | |
| Hank’s Buffered Salt Saline | Invitrogen | 14185 | |
| Phenol free, DMEM | Invitrogen | 21063 | |
| Fetal bovine serum | Aleken Biologicals | FBSS500 | |
| Penicillin/Streptomycin | Sigma-Aldrich | P0781-100ML | |
| Borosilicate Glass with filament | Sutter Instrument Co. | BF100-78-10 | |
| Gloss/Gloss non-reinforced silicone sheeting, 0.005" | Specialty Manufacturing Inc. | ||
| Dulbecco’s Phosphate Buffered Saline | Invitrogen | 14200 | |
| 35 mm glass bottom culture dishes (FluoroDish) | World Precision Instruments, Inc. | FD35-100 | |
| Braycote 804 Vacuum Grease | SPI Supplies | 05133A-AB |
Odniesienia
- Friedl, P., Wolf, K., Lammerding, J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 23, 55-64 (2011).
- Mejat, A., Misteli, T. LINC complexes in health and disease. Nucleus. 1, 40-52 (2010).
- Worman, H. J., Fong, L. G., Muchir, A., Young, S. G. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 119, 1825-1836 (2009).
- Caille, N., Tardy, Y., Meister, J. J. Assessment of strain field in endothelial cells subjected to uniaxial deformation of their substrate. Ann Biomed Eng. 26, 409-416 (1998).
- Lammerding, J. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 281, 25768-25780 (2006).
- Lammerding, J. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 170, 781-791 (2005).
- Lammerding, J. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 113, 370-378 (2004).
- Verstraeten, V. L., Ji, J. Y., Cummings, K. S., Lee, R. T., Lammerding, J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell. 7, 383-393 (2008).
- Brooks, S. V., Zerba, E., Faulkner, J. A. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol. 488, 459-469 (1995).
- Maniotis, A. J., Chen, C. S., Ingber, D. E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci. 94, 849-854 (1997).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone