Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Murine Superficial Lymph Node Surgery
W tym Artykule
Podsumowanie
To follow the progression of an immune response over time within the same mouse, lymph nodes can be sequentially removed by surgery. Here, we describe how this technique can be performed.
Streszczenie
In the field of immunology, to understand the progression of an immune response against a vaccine, an infection or a tumour, the response is often followed over time. Similarly, the study of lymphocyte homeostasis requires time course experiments. Performing these studies within the same mouse is ideal to reduce the experimental variability as well as the number of mice used. Blood withdrawal allows performance of time course experiments, but it only gives information about circulating lymphocytes and provides a limited number of cells1-4. Since lymphocytes circulating through the body and residing in the lymph nodes have different properties, it is important to examine both locations. The sequential removal of lymph nodes by surgery provides a unique opportunity to follow an immune response or immune cell expansion in the same mouse over time. Furthermore, this technique yields between 1-2x106 cells per lymph node which is sufficient to perform phenotypic characterization and/or functional assays. Sequential lymph node surgery or lymphadenectomy has been successfully used by us and others5-11. Here, we describe how the brachial and inguinal lymph nodes can be removed by making a small incision in the skin of an anesthetised mouse. Since the surgery is superficial and done rapidly, the mouse recovers very quickly, heals well and does not experience excessive pain. Every second day, it is possible to harvest one or two lymph nodes allowing for time course experiments. This technique is thus suitable to study the characteristics of lymph node-residing lymphocytes over time. This approach is suitable to various experimental designs and we believe that many laboratories would benefit from performing sequential lymph node surgeries.
Protokół
1. Preparation Before The Surgery
Mice were treated in accordance to the Canadian Council on Animal Care guidelines.
- Anesthetise mice by injection of ketamine/xylazine (150/300 mg/kg, i.p.) or by inhalation of isoflurane (2%, 1L oxygen). If available, isoflurane should be used as it allows for a better control of anaesthesia time and depth. Also, oxygen is administered at the same time which supports the physiological functions of the animal. Moreover, isoflurane is directly eliminated by the lungs and thus does not require hepatic or kidney metabolism.
- Inject buprenorphine (0.05 - 0.1 mg/kg, s.c.) or another analgesic.
- Apply ointment on the mouse eyes before surgery to avoid eye dryness (if isoflurane is used).
- Verify that the mouse is asleep by pinching its paw and rolling its tail. If the mouse does not react, proceed with the surgery.
2. The Surgery
It is possible to remove 4 of the superficial lymph nodes (LN) by surgery: the 2 inguinal and the 2 brachial. The isolation of LN is easier in young lean mice (6-8 weeks) since the presence of fatty tissue in older mice can interfere with LN isolation. However, this limitation is overcome with repeated practice. For the mice comfort, we harvest a maximum of 2 LNs at a time and when we perform time-course experiments, we harvest LNs every second day. Also, to prevent infection, antibiotics could be given to the mouse, but we do not and wounds are healing just fine. One needs to be aware that depending on the experimental design, the use of an antibiotic could influence the result observed (for example if a response to a bacterium is measured). Note that all instruments are sterilized after each use and aseptic conditions are maintained throughout the procedures.
- Put the mouse on its side.
- Localize the region where you will perform an incision, depending on the LN you want to harvest. (Figure 1).
- Apply chlorhexidine, or another disinfectant, to that region.
- Make a tiny incision with sharp scissors (about 5 mm) either on the back of the foreleg or above the hind paw near the abdomen (Figure 1).
- Remove the loose hairs you have cut with the forceps. If you prefer, you may shave the mouse before making the incision. However, it lengthens the procedure and we find that it is not necessary since, as shown here, it does not improve healing or decrease infection, which are both very rare.
- Stretch the incision with 2 forceps in order to see the LN. The incision might reach 10 mm. The LN appears grayish or darker than the surrounding fat.
- Pinch the fascia (the thin membrane covering the fat and tissue) on top of the LN with one forceps and pull lightly without breaking the surrounding tissue.
- Place the second forceps as far as possible underneath the LN. With the first forceps, break the fascia and remove the LN. If you have successfully harvested the LN, a stain of blood should appear at the location where the LN was previously located. Note that the LN sinks in isotonic solution. This simple test allows you to validate that you have extracted a LN and not fat tissue.
- Verify that no hairs are in the wound.
- Close the incision by sticking the 2 parts of skin together (the inside of the skin) and by stapling them with a clip. We use some Michel Clip with the applying forceps. Install one or 2 clips depending on the size of the incision. Most often, the clips fall off when the tissue heals.
3. Postoperative Care
- Add some wet food to the bottom of the cage so the mice can feed easily after the surgery.
- About 6 to 8 hours after the surgery, check the mice to ensure that they do not experience pain. Carefully observe their behaviour (posture, gait, interaction with congener and appearance of the fur). If the overall appearance of the mice is poor, administer a second dose of buprenorphine. By the next day the mice should regain their normal behaviour otherwise continue the administration of buprenorphine. The mice should never reach the endpoints previously determined and accepted by your Animal Care Council. You might want to seek advice and help from your animal care vivarium technician.
4. Lymph Node Handling
- In the laboratory, to obtain a single cell suspension, dissociate the LN as per your usual procedure. We press LN against frosted slides and routinely obtain 1-2 x 106 cells per LN. Alternatively, we prepare the LN for RNA extraction.
- Stain the cells as per your usual protocol. You should obtain expected LN flow cytometry profiles.
5. Representative Results
An example of forward and side scatter plot is shown for LNs harvested from an anesthetized mouse by surgery or from a sacrificed mouse (Figure 2). The profile and the percentages of cells in the live lymphocyte gate are the same demonstrating that LN surgery is an appropriate technique to harvest cells.
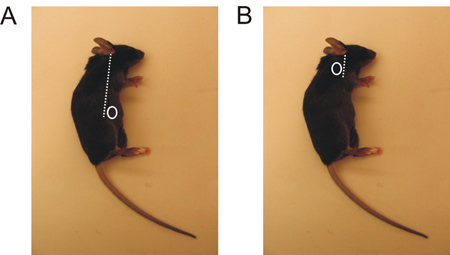
Figure 1. Inguinal and brachial LN localization in the mouse. A. The inguinal LN is located just above the hind paw, slightly toward the belly. B. The brachial LN is located behind the foreleg.
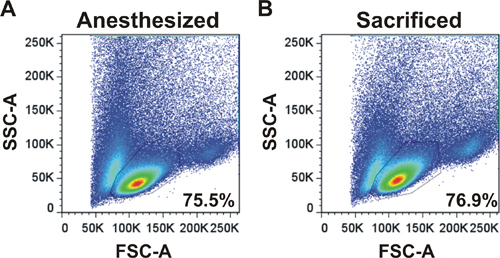
Figure 2. Flow cytometry profile of lymphocytes obtained following LN surgery. Forward and side scatter profiles are shown for LNs harvested from an anesthetized mouse by surgery (A) or from a sacrificed mouse (B). LNs were harvested, dissociated and analyzed on a flow cytometer. Percentages of cells in the live lymphocyte gate are shown.
Dyskusje
We have described a protocol of LN surgery which can apply to many experimental systems. Although very useful to follow immune responses or immune cell homeostasis, this technique has a few limitations. First, only four LNs can be harvested. Second, the immune response studied must be systemic or occur in the superficial inguinal or brachial (skin-draining) LNs. Finally, the number of cells harvested is influenced by the quality of the LN removal, a technical limitation which may contribute to experimental variability wh...
Ujawnienia
No conflicts of interest declared.
Podziękowania
We thank Sylvie Lesage and all lab members for critical reading of the protocol. This work was supported by a grant (MOP-77545) from the Canadian Institutes of Health Research (CIHR). Nathalie Labrecque is supported by a FRSQ Senior Scholarship and Mélissa Mathieu received a NSERC Alexander Graham Bell Canada Graduate Scholarship.
Materiały
| Name | Company | Catalog Number | Comments |
| Graefe Fine Pattern Premium Forceps | Harvard Apparatus | 52-2144 | Strongly Curved, 10 cm (4 in), 0.8 mm tip |
| Michel Clip Applying and Removing Forceps | Harvard Apparatus | 52-3779 | 12.5 cm (5 in) |
| Michel Clip 100 | Harvard Apparatus | 52-3746 | 7.5-1.75 mm |
| Temgesic (Buprenorphine hydrochloride) | Merck & Co. | ||
| Vetalar (Ketamine hydrochloride) | Bioniche Animal Health | DIN: 01989529 | |
| Rompun (Xylazine) | Bayer AG | DIN: 02169592 | |
| Isoflurane | Abbott Laboratories | DIN: 02032384 | |
| Hypotears eye ointment | Novartis AG | DIN: 02133288 | |
| Baxedin (Clorhexidine gluconate 2% in isopropyl alcohol 70%) | Omega Engineering, Inc. | L0000017 | DIN: 02251477 |
Odniesienia
- Zehn, D., Lee, S. Y., Bevan, M. J. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 458, 211-214 (2009).
- Vezys, V. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 457, 196-199 (2009).
- Zhou, X. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 33, 229-240 (2010).
- Wirth, T. C., Badovinac, V. P., Zhao, L., Dailey, M. O., Harty, J. T. Differentiation of central memory CD8 T cells is independent of CD62L-mediated trafficking to lymph nodes. J. Immunol. 182, 6195-6206 (2009).
- Rooke, R., Waltzinger, C., Benoist, C., Mathis, D. Targeted complementation of MHC class II deficiency by intrathymic delivery of recombinant adenoviruses. Immunity. 7, 123-134 (1997).
- Witherden, D. Tetracycline-controllable selection of CD4(+) T cells: half-life and survival signals in the absence of major histocompatibility complex class II molecules. J. Exp. Med. 191, 355-364 (2000).
- Labrecque, N. How much TCR does a T cell need. Immunity. 15, 71-82 (2001).
- Leignadier, J., Labrecque, N. Epitope density influences CD8+ memory T cell differentiation. PLoS One. 5, e13740 (2010).
- Leignadier, J., Hardy, M. P., Cloutier, M., Rooney, J., Labrecque, N. Memory T-lymphocyte survival does not require T-cell receptor expression. Proc. Natl. Acad. Sci. U.S.A. 105, 20440-20445 (2008).
- Obst, R., van Santen, H. M., Mathis, D., Benoist, C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J. Exp. Med. 201, 1555-1565 (2005).
- Bassett, J. D. CD8+ T-cell expansion and maintenance after recombinant adenovirus immunization rely upon cooperation between hematopoietic and nonhematopoietic antigen-presenting cells. Blood. 117, 1146-1155 (2011).
- Lacombe, M. H., Hardy, M. P., Rooney, J., Labrecque, N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J. Immunol. 175, 4400-4407 (2005).
- Leignadier, J., Rooney, J., Daudelin, J. F., Labrecque, N. Lowering TCR expression on naive CD8+ T cells does not affect memory T-cell differentiation. Immunol. Cell. Biol. 89, 322-325 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone