Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Labeling Stem Cells with Ferumoxytol, an FDA-Approved Iron Oxide Nanoparticle
W tym Artykule
Podsumowanie
We describe a technique for labeling and tracking stem cells with FDA-approved, superparamagnetic iron oxide (SPIO), ferumoxytol (Feraheme). This cellular imaging technique that utilizes magnetic resonance (MR) imaging for visualization, is readily accessible for long-term monitoring and diagnosis of successful or unsuccessful stem cell engraftments in patients.
Streszczenie
Stem cell based therapies offer significant potential for the field of regenerative medicine. However, much remains to be understood regarding the in vivo kinetics of transplanted cells. A non-invasive method to repetitively monitor transplanted stem cells in vivo would allow investigators to directly monitor stem cell transplants and identify successful or unsuccessful engraftment outcomes.
A wide range of stem cells continues to be investigated for countless applications. This protocol focuses on 3 different stem cell populations: human embryonic kidney 293 (HEK293) cells, human mesenchymal stem cells (hMSC) and induced pluripotent stem (iPS) cells. HEK 293 cells are derived from human embryonic kidney cells grown in culture with sheared adenovirus 5 DNA. These cells are widely used in research because they are easily cultured, grow quickly and are easily transfected. hMSCs are found in adult marrow. These cells can be replicated as undifferentiated cells while maintaining multipotency or the potential to differentiate into a limited number of cell fates. hMSCs can differentiate to lineages of mesenchymal tissues, including osteoblasts, adipocytes, chondrocytes, tendon, muscle, and marrow stroma. iPS cells are genetically reprogrammed adult cells that have been modified to express genes and factors similar to defining properties of embryonic stem cells. These cells are pluripotent meaning they have the capacity to differentiate into all cell lineages 1. Both hMSCs and iPS cells have demonstrated tissue regenerative capacity in-vivo.
Magnetic resonance (MR) imaging together with the use of superparamagnetic iron oxide (SPIO) nanoparticle cell labels have proven effective for in vivo tracking of stem cells due to the near microscopic anatomical resolution, a longer blood half-life that permits longitudinal imaging and the high sensitivity for cell detection provided by MR imaging of SPIO nanoparticles 2-4. In addition, MR imaging with the use of SPIOs is clinically translatable. SPIOs are composed of an iron oxide core with a dextran, carboxydextran or starch surface coat that serves to contain the bioreactive iron core from plasma components. These agents create local magnetic field inhomogeneities that lead to a decreased signal on T2-weighted MR images 5. Unfortunately, SPIOs are no longer being manufactured. Second generation, ultrasmall SPIOs (USPIO), however, offer a viable alternative. Ferumoxytol (FerahemeTM) is one USPIO composed of a non-stoichiometric magnetite core surrounded by a polyglucose sorbitol carboxymethylether coat. The colloidal, particle size of ferumoxytol is 17-30 nm as determined by light scattering. The molecular weight is 750 kDa, and the relaxivity constant at 2T MRI field is 58.609 mM-1 sec-1 strength4. Ferumoxytol was recently FDA-approved as an iron supplement for treatment of iron deficiency in patients with renal failure 6. Our group has applied this agent in an “off label” use for cell labeling applications. Our technique demonstrates efficient labeling of stem cells with ferumoxytol that leads to significant MR signal effects of labeled cells on MR images. This technique may be applied for non-invasive monitoring of stem cell therapies in pre-clinical and clinical settings.
Protokół
1. Day 1
1) Plate cells
- Plate hMSC in a T75 flask at a confluency of 80% at least 18-24 hours prior to labeling. Refer to Table 1 for instruction for alternate vessels.
2. Day 2
2) Prepare labeling solution. This preparation will label one (1) T75 flask at 80% confluency with a concentration of 400 μg Fe/ml. Refer to Table 1 for instruction for alternate vessels.
- Create a solution (solution 1) by mixing 1 ml of serum-free media and 93.1 μl of ferumoxytol (stock: 30 mg/ml) in a 15 ml conical tube.
- Create a second solution (solution 2) by mixing 1 ml of serum-free media and 7 μl of protamine sulfate (stock: 10 mg/ml) in a second 15 ml conical tube.
- Allow each solution to rest for 5 minutes.
- Add solutions 1 and 2 together. Gently mix the new labeling solution. Allow the solution to rest for 5 minutes to permit USPIO-protamine sulfate complexes to form.
- Add 5 ml of serum-free media to the mixed 2 ml of SPIO/protamine sulfate solution for a final volume of 7 ml.
3) Prepare cells for labeling
- Aspirate the media from cells.
- Gently wash cells once with 2-3 ml of pre-warmed Mg/Ca-free D-PBS or serum-free media to rinse away residual serum proteins and or other media components that could impair contrast agent uptake and labeling efficiency. Aspirate out the rinse fluid.
4) Label cells
- Add the complete 7 ml of labeling solution to cells.
- Place cells into an incubator (37°C/ 5% CO2) and allow the cells to incubate in the labeling solution for 4 hours.
- Add 700 μl of FCS to the labeling solution of the cells to achieve a final serum concentration of 10%. Serum is added to re-establish the enriched environment of which the cells are accustomed, and to assist in minimizing cell death that could result from an abrupt shift to 24 hour exposure to a serum-free environment.
- Allow the cells to incubate with the labeling solution for an additional 20 hours.
3. Day 3
5) Prepare labeled cells
- Aspirate the labeling solution from cells.
- Gently rinse cells with 2-3 ml of pre-warmed Mg/Ca-free D-PBS. Aspirate out the PBS. It is important to use Mg/Ca-free PBS as the Mg and Ca will impair the efficiency of Trypsin in the next step.
- Add 2 ml of pre-warmed 0.05% Trypsin to the cells and tilt the flask back and forth to ensure the entire surface of the flask is covered with a thin layer of Trypsin. Place the cells in an incubator (37°C/ 5% CO2) and allow the cells to rest for 5-7 minutes or until cells begin to detach from the plate. It may be necessary to confirm detachment under a microscope. Gently tap the sides of the flask if necessary to facilitate cell-surface detachment.
- Add 4 ml of pre-warmed complete media to the flask to neutralize the Trypsin. Gently rinse the flask by pipetting the Trypsin/media/cell solution up and down several times. Collect the entire solution in a 15 ml conical tube. Centrifuge the cell solution at 400 RCF for 5 minutes.
- Carefully aspirate the supernatant without disturbing the cell pellet. Resuspend the cells in 5 ml of media (serum containing or serum-free). Centrifuge the cell solution at 400 RCF for 5 minutes.
- Repeat the cell wash described in step 5.5. In total, cells will be rinsed three times to remove residual, free contrast agent in media and on the surface of cells.
- Count cells at this point to attain the most accurate cell count, as cells will be lost during previous washing. Perform a viability test of cells. Cells are now ready for subsequent analysis and or experimental application. MR imaging can be performed with T1-w and T2-w parameters, because although ferumoxytol is a T2-w contrast agent, ferumoxytol also exhibits T1 effects. Representative T2-w MRI parameters that can be used to image ferumoxytol labeled cells include: FSE or SE_Multislice sequences with a TR of 2000-2500 ms and a TE of 60-80 ms. To acquire a T1-w image, decrease the TE value on a FSE or SE_Multislice sequence or use a GRE sequence with a high TR and low TE. Figure 4 demonstrates a potential in vivo application for labeled stem cell imaging.
4. Representative Results:
Labeled cells demonstrate a significant darkening or negative contrast effect on T2-weighted MR images and a brightening or positive contrast effect on T1-weighted MR images (Figure 2). Longitudinal MR imaging and viability tests performed 5 days post labeling demonstrated no significant MR signal and no significant impact on viability compared to unlabeled controls (data not shown).
Labeled stem cells can subsequently be differentiated into various cell types or intravenously injected for in vivo investigation.
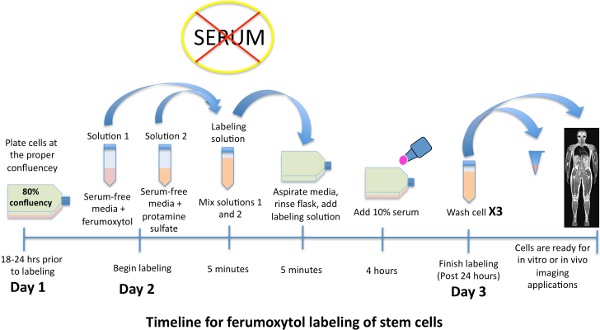
Figure 1. Schematic time line for labeling stem cells with iron oxide nanoparticle, ferumoxytol.

Figure 2. MR images of sagittal cross sections thrrough eppendorf tubes with cells in a cell pellet. A) Unlabeled controls. B) Labeled cells demonstrating a significant T2 or negative contrast agent effect on T2-weighted FSE or SE_Multislice imaging and a T1 or positive contrast agent effect on the T1-weighted GRE sequence.
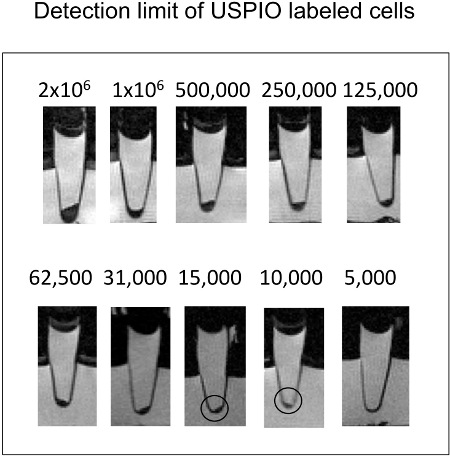
Figure 3. Sagittal cross sections of eppendorf tubes with cells in cell pellets. As little as 10,000 ferumoxytol labeled cells can be detected via T2-w MR imaging.
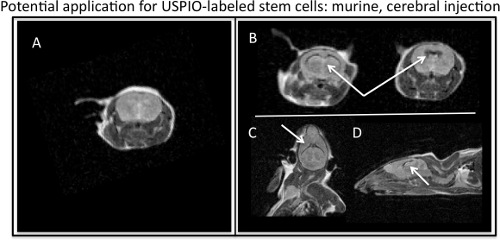
Figure 4. MR visualization of ferumoxytol-labeled stem cells injected into murine cerebral ventricles. A) Axial, MR image of murine barin with no injection of USPIO-labeled stem cells. B) Axial, C) coronal, and D) sagittal MR images depicting the T2, negative contrast effect of USPIO-labeled stem cells (white arrows) injected in a murine brain.

Table 1. Quantity adaptions for labeling cells in alternate vessels.
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Improving the efficacy of stem cell engraftments is critical for the advancement of regenerative medicine. A non-invasive visualization technique for stem cells in vivo significantly enhances our ability to understand mechanisms that lead to successful engraftment outcomes. Magnetic labeling for MR visualization, such as the procedure we have demonstrated, allows in vivo tracking of stem cells with MR imaging. Magnetically labeled stem cells have previously been transplanted into target tissues and have...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
All contributors to this study offer no disclosures.
Podziękowania
This work was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases: 3R01AR054458-02S2.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| D-MEM High Glucose | Sigma-Aldrich | D5648 | Or other base medium for desired stem cell line to be used |
| D-PBS (Ca++, Mg++ free) | GIBCO, by Life Technologies | 14190-144 | |
| Trypsin-EDTA 0.05% | Invitrogen | 25300-120 | |
| Fetal Bovine Serum (FBS) | Hyclone | SH30071.03 | |
Ferumoxytol (Feraheme) | AMAG | 59338-0775-01 | |
| Protamine Sulfate | APP Pharmaceuticals | 22930 |
Odniesienia
- Narsinh, K. H., Plews, J., Wu, J. C. Comparison of human induced pluripotent and embryonic stem cells: fraternal or identical twins? Mol Ther. 19, 635-638 (2011).
- Bulte, J. W. In vivo MRI cell tracking: clinical studies. AJR. Am. J. Roentgenol. 193, 314-325 (2009).
- Henning, T. D., Boddington, S., Daldrup-Link, H. E. Labeling hESCs and hMSCs with Iron Oxide Nanoparticles for Non-Invasive in vivo Tracking with MR Imaging. J. Vis. Exp. (13), e685-e685 (2008).
- Tallheden, T., Nannmark, U., Lorentzon, M. In vivo MR imaging of magnetically labeled human embryonic stem cells. Life. Sci. 79, 999-1006 (2006).
- Jung, C. W., Jacobs, P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging. 13, 661-674 (1995).
- Coyne, D. W. Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert. Opin. Pharmacother. 10, 2563-2568 (2009).
- Li, Z., Suzuki, Y., Huang, M. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 26, 864-873 (2008).
- Metz, S., Bonaterra, G., Rudelius, M. Capacity of human monocytes to phagocytose approved iron oxide MR contrast agents in vitro. Eur. Radiol. 14, 1851-1858 (2004).
- Nedopil, A., Klenk, C., Kim, C. MR signal characteristics of viable and apoptotic human mesenchymal stem cells in matrix-associated stem cell implants for treatment of osteoarthritis. Invest. Radiol. 45, 634-640 (2010).
- Kraitchman, D. L., Heldman, A. W., Atalar, E. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 107, 2290-2293 (2003).
- Stuckey, D. J., Carr, C. A., Martin-Rendon, E. Iron particles for noninvasive monitoring of bone marrow stromal cell engraftment into, and isolation of viable engrafted donor cells from, the heart. Stem Cells. 24, 1968-1975 (2006).
- Henning, T. D., Sutton, E. J., Kim, A. The influence of ferucarbotran on the chondrogenesis of human mesenchymal stem cells. Contrast. Media. Mol. Imaging. 4, 165-173 (2009).
- Arbab, A. S., Yocum, G. T., Kalish, H. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 104, 1217-1223 (2004).
- Nedopil, A. J., Mandrussow, L. G., Daldrup-Link, H. E. Implantation of Ferumoxides Labeled Human Mesenchymal Stem Cells in Cartilage Defects. J. Vis. Exp. (38), e1793-e1793 (2010).
- Arbab, A. S., Yocum, G. T., Wilson, L. B. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 3, 24-32 (2004).
- Babic, M., Horak, D., Trchova, M. Poly(L-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug Chem. 19, 740-750 (2008).
- Golovko, D. M., T, H. enning, Bauer, J. S. Accelerated stem cell labeling with ferucarbotran and protamine. Eur. Radiol. 20, 640-648 (2010).
- Lu, M., Cohen, M. H., Rieves, D. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am. J. Hematol. 85, 315-319 (2010).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone