Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Fabrication and Use of MicroEnvironment microArrays (MEArrays)
W tym Artykule
Podsumowanie
A combinatorial functional screening method for gaining insights into the impacts of the molecular composition of microenvironments on cellular functions is described. The method takes advantage of existing microarray-based technologies to generate arrays of defined combinatorial microenvironments that support cell adhesion and functional analysis.
Streszczenie
The interactions between cells and their surrounding microenvironment have functional consequences for cellular behaviour. On the single cell level, distinct microenvironments can impose differentiation, migration, and proliferation phenotypes, and on the tissue level the microenvironment processes as complex as morphogenesis and tumorigenesis1. Not only do the cell and molecular contents of microenvironments impact the cells within, but so do the elasticity2 and geometry3 of the tissue. Defined as the sum total of cell-cell, -ECM, and -soluble factor interactions, in addition to physical characteristics, the microenvironment is complex. The phenotypes of cells within a tissue are partially due to their genomic content and partially due to the combinatorial interactions with the microenviroment. A major challenge is to link specific combinations of microenvironmental components with distinctive behaviours.
Here, we present the microenvironment microarray (MEArray) platform for cell-based functional screening of interactions with combinatorial microenvironments4. The method allows for simultaneous control of the molecular composition and the elastic modulus, and combines the use of widely available microarray and micropatterning technologies. MEArray screens require as few as 10,000 cells per array, which facilitates functional studies of rare cell types such as adult progenitor cells. A limitation of the technology is that entire tissue microenvironments cannot be completely recapitulated on MEArrays. However, comparison of responses in the same cell type to numerous related microenvironments, for instance pairwise combinations of ECM proteins that characterize a given tissue, will provide insights into how microenvironmental components elicit tissue-specific functional phenotypes.
MEArrays can be printed using a wide variety of recombinant growth factors, cytokines, and purified ECM proteins, and combinations thereof. The platform is limited only by the availability of specific reagents. MEArrays are amenable to time-lapsed analysis, but most often are used for end point analyses of cellular functions that are measureable with fluorescent probes. For instance, DNA synthesis, apoptosis, acquisition of differentiated states, or production of specific gene products are commonly measured. Briefly, the basic flow of an MEArray experiment is to prepare slides coated with printing substrata and to prepare the master plate of proteins that are to be printed. Then the arrays are printed with a microarray robot, cells are allowed to attach, grow in culture, and then are chemically fixed upon reaching the experimental endpoint. Fluorescent or colorimetric assays, imaged with traditional microscopes or microarray scanners, are used to reveal relevant molecular and cellular phenotypes (Figure 1).
Protokół
1. Printing Substrata Preparation
The decision to use polydimethylsiloxane (PDMS)-coated or polyacrylamide (PA)-coated slides depends on the important parameters of the experimental design. The elastic modulus of both polymers can be tuned to mimic the stiffnesses of different tissues by altering the base/cure ratio of PDMS, and the acrylamide/bis-acrylamide ratio of PA. PDMS can mimic stiffer tissues in the range of 1-10MPa (e.g. cartilage, cornea, and arterial walls), and PA can mimic softer tissues in the range of 100Pa-100kPa (e.g. breast, brain, liver, and prostate) 5. PDMS is inexpensive, easy to prepare, and the geometry of the printed features will be identical to the head of the printing pins. Thus the size and shape of the features can be precisely controlled using pins with different tip geometries. PDMS is more hydrophobic than PA, which causes some challenges during the cell handling and immunostaining steps, and may be incompatible with some cell lines. Because PA is a hydrogel and a native non-fouling surface, cells will only attach to spots where there are proteins that support cell adhesion. The geometry of the printed features on PA gels do not precisely follow the geometry of the pinhead; usually they become circles due to diffusion, irrespective of the pinhead geometry that is used. Printing contact time and pin diameter parameters can be empirically determined for optimal feature size on PA gels.
Polydimethylsiloxane (PDMS)
- In a disposable plastic cup combine Sylgard 184 silicone elastomer base with the curing agent at a 10:1 ratio, mix vigorously with a wooden or plastic tongue depressor then degas in a room temperature vacuum bell for 30 min.
- Center a standard microscope slide on the vacuum actuated chuck of a spin coater, then drizzle 0.5 ml of the mixed elastomer polymer onto the center of the slide surface. Spin at 6,000 rpm for 60 sec.
- Cure the PDMS-coated slides in a 70 °C oven or on a digital hot plate (protected from dust) for 4 hr to overnight.
- Cured slides can be used immediately, or stored for several months in a slide box that is sealed within a plastic Ziploc bag and kept in a drawer. The PDMS attracts dust so it must be well protected from room air circulation.
- Note: Nitrile or other non-latex gloves must be worn when working with the PDMS elastomer kit. Incidental contact with latex gloves will inhibit PDMS polymerization.
Polyacrylamide (PA)
- NaOH etching: Place slides on heat block at 80 °C. Add 1 ml 0.1N NaOH on each slide, making sure to cover the entire slide surface. Let the NaOH evaporate (a white film should form on the slide surface). Since the PA gel can only attach firmly on NaOH etched surfaces, the PA gel will detach during drying out step if the entire slide surface is not covered by NaOH. If the slide surface was not covered completely, repeat by adding 1 ml 0.1N NaOH. Slides can then be stored at room temperature (RT) for several days. Note: An alternative to NaOH etching is to ozone- or plasma-clean the slides.
- 3-Aminopropyltriethoxysilane (APES) coating: In a fume hood, place slides in a 15 ml dish, and add 300 μl APES on each NaOH etched slide. Let the APES react with the NaOH slides for 5 min. Exceeding this time will cause difficulty in washing out unreacted APES reagent. Wash out APES thoroughly with deionized water two to three times on both sides of the slides. If the washing is not complete, APES will be oxidized by Glutaraldehyde to form a brown deposit on slides in step 1.8.
- Glutaraldehyde oxidation: Aspirate all the solutions from the slide surfaces. In each 15 cm dish, add 25 ml 0.5% glutaraldehyde in PBS. React for 30 min in a dark area. After 30 min, aspirate all the glutaraldehyde and use non-lint laboratory wipes (e.g. Kimwipes) to carefully dry the slides. Slides can then be stored at RT for up to one day.
- Gel preparation: After preparing PA mixtures including acrylamide, bis-acrylamide and ddH2O in according with the table below, degas for 30 min, and then place PA mixtures on ice to slow down polymerization. Add APS and TEMED and mix well right before making the gels. Pipet PA mixtures onto the slide surfaces and place 24 mm x 50 mm, number 1 coverslips on top of the PA. Avoid pressing coverslip and glass slide together and avoid bubble formation. For gels >40,000 Pa use 100 μl, for other gels use 350 μl.
| Desired modulus (Pa) | Acrylamide% | Bis-acrylamide% | Acrylamide from 40% stock (ml) | Bis-Acrylamide from 2% stock (ml) | Deionized water (ml) | APS (μl) | TEMED (μl) |
| 480±160 | 3 | 0.06 | 0.75 | 0.3 | 8.95 | 100 | 10 |
| 4470±1190 | 5 | 0.15 | 1.25 | 0.75 | 8 | 100 | 10 |
| 40,400±2390 | 8 | 0.48 | 2 | 2.4 | 5.6 | 100 | 10 |
Adapted from 6,7.
- Let the PA gel polymerize for 2 hr, and then remove coverslips under deionized water.
- Wash PA gel slides in large Coplan jars in water overnight (~8 hr) to remove unreacted acrylamides.
- Dry slides in a 37 °C oven for 2-4 hr or until PA gel completely hardens.
- PA gel-slides can be stored at 4 °C for one month in a sealed slide box.
2. Protein Master Plate Preparation
- All proteins should be aliquoted in stocks of 10X solutions in the buffers recommended by the provider and stored at -80 °C. Most ECM proteins are soluble in deionized water, but the pH may need to be adjusted with drops of acetic acid. Most growth factors, cytokines, and recombinant receptor extracellular domains are prepared in PBS with BSA, but manufacturer conditions will vary. Filter the protein aliquots through a 0.45 μm 4-mm nylon syringe filter (Nalgene) prior to storage.
- Design a master plate in accordance with desired protein combinations and dilutions. Adherent cells usually rely on the presence of at least one compatible ECM to mediate cell adhesion, but antibodies to cell surface epitopes can also mediate attachment sometimes. It is a good idea to add free FITC dye or a fluorphore-conjugated protein to at least one well so that arrays can be easily oriented later.
- Prepare the master plate by diluting the protein combinations with printing buffer composed of 100 mM Tris-acetate/20% glycerol/0.05% Triton-100X pH 5.2. Typically each well of a 384-well plate contains no more than 10 μl.
- Record the contents of each well in each master plate in a tab delimited data base file and provide each master plate with a unique identification number. A six-digit date followed by the designer's initials often serves the purpose (MMDDYYinitial). Because the well volumes are small, protein aliquots can be used efficiently to generate a large numbers of replicate plates. It is recommended that master plates are stored at -80 °C and each master plate should undergo no more than two freeze-thaw cycles.
3. MEArray Printing
- MEArrays can be printed with most conventional microarray printing robots. Quill pin printers that use either silicone or stainless steel pins work well, but protein viscosity can be problematic. Capillary printers are ideal microarray printing robots for this application, as they work well with viscous protein solutions.
- To attain good statistical power within an array, 10 to 12 replicate spots of each microenvironment is recommended. Such a design will allow comparison of activity in one microenvironment relative to another in the same array using simple T-test statistics. Dunnette's test can be used to compare activity in a control environment with other microenvironments. This design works best when a functional phenotype has been associated with the control microenvironment before performing the MEArray experiments.
- Humidity should be maintained around 50%. Humidity control is important because a low humidity can dry the solution inside the pins or in the wells of the master plate causing inefficient deposition on the printing substrata. Humidity can be controlled effectively by draping the robot with non-porous plastic sheeting and using both a humidifier and a de-humidifier set to maintain 50% humidity. Cooled printing plattens can be useful for preserving some proteins, but caution must be taken to avoid condensation from forming on the slides.
- Each printed array should be labeled with freezer-proof slide labels encoded with a serial number that consists of the master plate's identifier followed by a three digit number (MMDDYYinitial-nnn). As every array is used or distributed, details of their experimental treatments should be maintained in a database. Tracking the dates of printing and the numbers of freeze-thaw cycles of the master plates will help to identify the optimal conditions for maintaining reproducibility.
- Printed MEArrays should be stored in sealed slide boxes at -20 °C for no more than one month. Reproducibility noticeably declines thereafter.
4. Culturing Mammalian Cells on MEArrays for Functional Analysis
- Attach culture chambers: To limit the volume of media and numbers of cell required culture on the MEArrays, a plastic chamber is fitted to surround the printed array. For many arrays, a single chamber from a 2-chamber slide (Nunc) that contains an area of 4.2 cm2 can be used. Remove the chambers from the manufactured chamber slide and cut the chambers in half with a razor blade. Use a 3 ml syringe to apply a thin bead of aquarium silicone (DAP) to the edge of a chamber and press on the surface of a MEArray. Avoid placing the applied aquarium silicone chamber on the array features.
- Blocking and rinsing: MEArrays need to be well rinsed to remove unreacted monomers, which can be toxic to cells. If PDMS-coated slides were used, then the regions in between the printed features first need to be blocked with a non-fouling coating to prevent cell adhesion; incubate the arrays in 1% Pluronic F108 (BASF) in water or 2 %BSA in water for 15 min under vacuum. PA gel slides do not require a blocking step. In all cases, rinse arrays with cell culture media three times for five minutes (media choice depends upon the cells used, but use of antibiotics is recommended regardless of media or cells). PA gels require additional 30 min incubation in media to rehydrate the gel.
- Cell attachment: Four to five arrays can fit inside of a single 15 cm sterile Petri dish. Cover the Petri dish with a lid to keep the arrays sterile. Add half of the final media volume to the MEArray by adding the cells in media to a final concentration of 10,000 to 1,000,000 cells/ml. Cells will attach to the printed features at different rates depending on the composition of the printed microenvironment. Check for uniform attachment by viewing the arrays through an inverted stage microscope in 15 to 20 min intervals. By gently shaking the MEArrays back and forth, cells attaching in a patterned manner can be distinguished from the floating, unattached cells.
- Removal of unbound cells: On PA-coated MEArrays, the unbound cells can be aspirated and the media can be replaced with an appropriate volume. On PDMS-coated MEArrays, the media can never be completely removed from the well because the cells dry out and die almost immediately. Thus on PDMS-coated MEArrays, the unbound cells must be removed by a process of successive exchanges of half of the volume of media until any unbound cells are removed, as determined by microscopic inspection. The de-wetting effect of PDMS is less prominent when serum-containing media is used compared to defined media, and when BSA is used to block the unprinted areas compared to Pluronics F108.
- Cells can be cultured on MEArrays placed inside of 15 cm Petri dishes for many days with normal media changes. Media changes on PDMS slides must be done with successive changes of half of the media volume.
- Common fixatives, such as paraformaldehyde and methanol/acetone, are compatible with MEArray systems. When staining cells on PA-coated MEArrays, fixatives can be added and washed away just as they would be in a conventional staining procedure. However when staining cells on PDMS-coated MEArrays, the surface must remain wet even during the fixation. Aspirate half of the media and replace with a fixative. Repeat the process a few times until the well is filled with a majority of fixative. After fixation, the fixative is gradually replaced in the same manner with blocking buffer that is appropriate for the next step of analysis.
- Immunostaining is commonly used to analyze cellular functions. Staining routines will vary, but when working on the PDMS MEArrays, one needs to perform every washing and aspiration step as above, gradually changing the solutions and never allowing the surface to de-wet. De-wetting will cause artifacts in staining.
- The chambers can be removed with the aid of a razor blade. Coverslips can be mounted on top of stained MEArrays using Fluoromount-G (Southern Biotech). Detection can be performed with most multicolor fluorescence microarray scanners or on confocal microscopes with motorized tiled image acquisition modes.
5. Representative Results
An example of patterned protein deposition on a printed PDMS-coasted MEArray using a square-tipped silicon pins on a quill pin microarray-printing robot is shown in Figure 2. Deposition of various proteins that are printed can be verified by immunofluorescence using antibodies (Figure 2A). Dilutions of the protein solutions in the master plate are reflective of the amount (fluorescent intensity) that is deposited on the printing substrata surface (Figure 2B). Cells should attach to the printed features in an obvious patterned manner (Figure 2C).
An example of an MEArray experiment showing that inverse dilutions of two microenvironment proteins elicited specific keratin expression profiles in a protein concentration-dependent manner in a human multipotent mammary epithelial progenitor cell line (D920 cells), is shown in Figure 3. Bubble plots are useful for determining whether specific phenotypes are imposed upon cells on replicate features of a dilution series. For instance, if a particular molecule in a microenvironment causes a distinct phenotype, once the instructive component has been diluted enough into a background of a neutral ECM the phenotype should change or disappear. Immunofluorescence detection of keratin 8 and keratin 14 intermediate filament proteins was performed with an Axon 4200a (Molecular Devices) microarray scanner. Twelve replicate dilution series were printed on each MEArray, and the log2 ratio of keratin 8 to keratin 14 mean fluorescence intensity was graphed as a bubble plot to give a realistic idea of variation and reproducibility of the signal. Shown is data from an MEArray that was fixed after cells had attached and unbound cells were washed away (Figure 3A), and after 24 hr of culture (Figure 3B). For this relatively small analysis, a one-way ANOVA was used to determine variance from the mean signal at each time point, and grouped two-tailed T-tests were used to determine whether the different dilutions of type I collagen and recombinant human P-cadherin caused changes in keratin expression. There was no variation from the mean among cells on the features just after attachment; however, there were significant differences in keratin expression among cells after 24 hr of exposure to the different microenvironments. T-tests verified that high type I collagen concentrations elicited higher keratin 8 expression, whereas high P-cadherin concentrations elicited a strong keratin 14 signal after 24 hr. This result was consistent with previous reports that P-cadherin-containing microenvironments will impose of K14-expressing myoepithelial phenotype on bi-potent mammary progenitor cells4.
An example of an entire scanned MEArray printed on a 40,000Pa PA gel is shown in Figure 4.

Figure 1. A flow chart of the MEArray procedure. First, the printing substrata are prepared either with PDMS or PA. Second, the master plates are prepared and annotated in a database. Third, the MEArrays are printed and encoded with serial numbers. Fourth, culture chambers are attached, surfaces are blocks and/or rinsed, then cells are allowed to attach and unbound cells are washed away. Fifth, cells can be treated with staining or bio-assay after a period of incubation based on experimental design. Finally, Images of MEArray can be obtained and analyzed with suitable scanner and software.
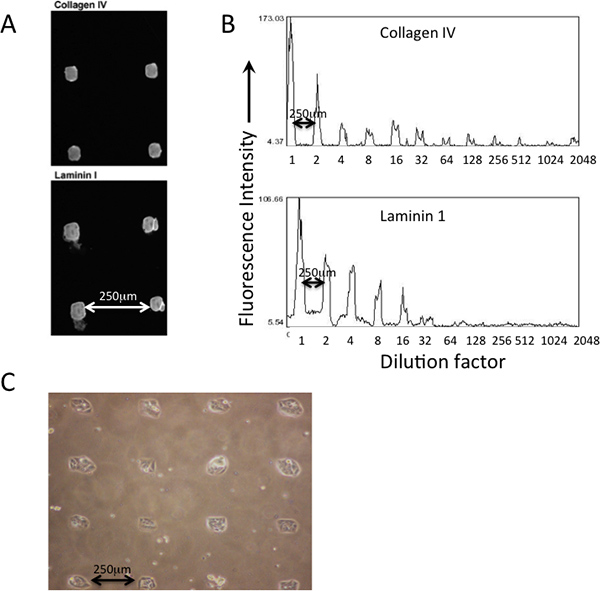
Figure 2. Deposition and relative abundance of printed proteins can be verified with immunostaining prior to cell attachment. A) Antibodies that recognized type IV collagen and laminin-111 were used to verify their presence in printed features of an MEArray. B) Using an average pixel intensity analysis feature in NIH ImageJ software, the relative abundance of the two proteins across a series of dilutions, starting from a 200 μg/ml protein solution, can be qualitatively assessed. C) Phase micrograph of D920 cells attached to square-shaped features of a printed PDMS-coated MEArray.
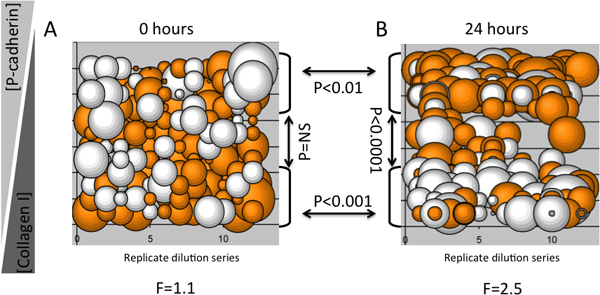
Figure 3. An example of an MEArray analysis using changes in keratin expression in a multipotent progenitor cell line as a functions of time and microenvironment. Each bubble represents ratios of keratin 8 and keratin 14 protein levels from 10-15 cells attached to a feature in a MEArray. Expression was determined with immunofluorescent probes. A) Shows the keratin ratios in cells just after attachment, and B) shows the keratin ratios after 24 hr on an array that was plated in parallel. The maximum concentration of both proteins was 200 μg/ml and diluted 2-fold. The diameter of a bubble represents the magnitude of the log2 ratio of keratin 8 and keratin 14 mean intensity, and the orange and white color-coding indicates values >0 and <0, respectively. F-values for one-way ANOVA and P-values from T-tests, and brackets with arrows identifying the populations compared, are shown.
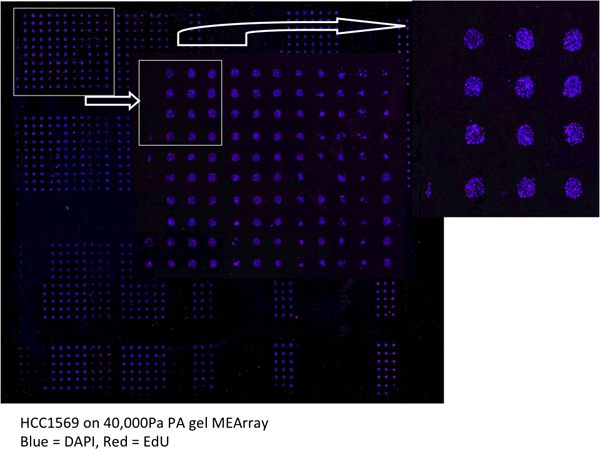
Figure 4. An example of an MEArray scan acquired using a tiled acquisition mode on a laser scanning confocal microscope. HCC1569 cells we allowed to incorporate the DNA analog EdU for 4 hr prior to fixation. DAPI (blue) and EdU (red) are shown.
Dyskusje
The MEArray method presented here enables functional analyses of cell and combinatorial microenvironment interactions4. MEArray analysis combines use of basic micropatterning technologies, cell biology, and microarray printing robots and analysis devices that are available in many multiuser facilities. MEArray screens are compatible with most adherent cell types, though serum-free media formulations may need to be adjusted in some cases to include BSA or <1% serum, which can improve attachment. This method ...
Ujawnienia
No conflicts of interest declared.
Podziękowania
ML is supported by the NIA (R00AG033176 and R01AG040081) and by Laboratory Directed Research and Development, US Department of Energy contract# DE-AC02-05CH11231.
Materiały
| Name | Company | Catalog Number | Comments |
| Glass slides 25 mm x 75 mm | VWR | 48311-600 | |
| Glass coverslips (no.1) 24 mm x 50 mm | VWR | 48393-241 | |
| Staining dish (or Coplan jar) | VWR | 25461-003 | |
| Petri dishes (15 cm) | BD Falcon | 351058 | |
| NaOH (1.0N) | Sigma-Aldrich | S2567 | |
| APES (>98% (3-Aminopropyl)triethoxysilane) | Sigma-Aldrich | A3648 | |
| Glutaraldehyde | Sigma-Aldrich | G7651 | 50% in water |
| APS (>98% Ammonium Persulfate) | Sigma-Aldrich | A3678 | Prepare 10% working solution with ddH2O |
| TEMED (N,N,N',N'-Tetramethylethylenediamine) | Sigma-Aldrich | T9281 | |
| Acrylamide (40%) | Sigma-Aldrich | A4058 | |
| Bis-Acrylamide (2% w/v) | Fisher BioReagents | BP1404-250 | |
| 0.45 μm Syringe filter 4-mm nylon | Nalgene | 176-0045 | |
| FITC | Sigma-Aldrich | F4274 | |
| PDMS (polydimethylsiloxane) | Dow Corning | 3097358-1004 | Sylgard 184 Elastomer kit via Ellsworth Adhesives |
| 2-chamber slides | NUNC | 177380 | |
| Pluronic F108 | BASF | 30089186 | |
| Aquarium sealant | Dow Corning | DAP 00688 | |
| Fluormount-G | Southern Biotech | 0100-01 | |
| Disposable plastic cups | |||
| Tongue depressors | |||
| Nitrile gloves | |||
| Plastic microscope slide boxes | |||
| Spin coater | WS-400B-6NPP/LITE | Laurell Technologies Corporation | |
| Oven | |||
| Digital hotplate | |||
| 384-well plates | A brand appropriate for the microarray robot | ||
| Microarray printing robot | |||
| Inverted phase and fluorescence microscope | |||
| Axon microarray scanners | Molecular Devices | Multiple configurations exist |
Odniesienia
- Bissell, M. J., Labarge, M. A. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment. Cancer Cell. 7, 17-23 (2005).
- Engler, A. J., Sen, S., Sweeney, H. L., Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell. 126, 677-689 (2006).
- McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K., Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 6, 483-495 (2004).
- LaBarge, M. A. Human mammary progenitor cell fate decsions are products of interactions with combinatorial microenvironments. Integrative Biology. 1, 70-79 (2009).
- Kim, H. N. Patterning Methods for Polymers in Cell and Tissue Engineering. Annals of biomedical engineering. , (2012).
- Boudou, T., Ohayon, J., Picart, C., Pettigrew, R. I., Tracqui, P. Nonlinear elastic properties of polyacrylamide gels: implications for quantification of cellular forces. Biorheology. 46, 191-205 (2009).
- Tse, J. R., Engler, A. J. Preparation of hydrogel substrates with tunable mechanical properties. Current protocols in cell biology. Chapter 10, Unit 10 (2010).
- Flaim, C. J., Chien, S., Bhatia, S. N. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods. 2, 119-125 (2005).
- Soen, Y., Mori, A., Palmer, T. D., Brown, P. O. Exploring the regulation of human neural precursor cell differentiation using arrays of signaling microenvironments. Mol. Syst. Biol. 2, 37 (2006).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone