Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Ex Vivo Assessment of Contractility, Fatigability and Alternans in Isolated Skeletal Muscles
W tym Artykule
Podsumowanie
We describe a method to directly measure muscle force, muscle power, contractile kinetics and fatigability of isolated skeletal muscles in an in vitro system using field stimulation. Valuable information on Ca2+ handling properties and contractile machinery of the muscle can be obtained using different stimulating protocols.
Streszczenie
Described here is a method to measure contractility of isolated skeletal muscles. Parameters such as muscle force, muscle power, contractile kinetics, fatigability, and recovery after fatigue can be obtained to assess specific aspects of the excitation-contraction coupling (ECC) process such as excitability, contractile machinery and Ca2+ handling ability. This method removes the nerve and blood supply and focuses on the isolated skeletal muscle itself. We routinely use this method to identify genetic components that alter the contractile property of skeletal muscle though modulating Ca2+ signaling pathways. Here, we describe a newly identified skeletal muscle phenotype, i.e., mechanic alternans, as an example of the various and rich information that can be obtained using the in vitro muscle contractility assay. Combination of this assay with single cell assays, genetic approaches and biochemistry assays can provide important insights into the mechanisms of ECC in skeletal muscle.
Wprowadzenie
Skeletal muscles attach to bones of the skeleton and generate contractile forces under the control of the central nervous system. Excitation-Contraction coupling (ECC) refers to the process of converting an electrical stimulus to a mechanical response. Ca2+ signaling is an essential component of the contractile function in skeletal muscle. Effective Ca2+ mobilization from sarcoplasmic reticulum (SR) is an important component for ECC in muscle cells1, 2, and changes in intracellular Ca2+ signaling underlie the corresponding contractile dysfunction in a number of muscle diseases3-5. Proper assessment of muscle contractility is essential and complimentary to Ca2+ imaging and other assays to gain insights into skeletal muscle function, not just at the contractile level, but also at the kinetic level. Force and speed can also be obtained to inform the important property of muscle power and the status of the ECC process under different physiological and pathophysiological conditions.
This fecund field of research has a very rich history and many theories of muscle contraction appeared over two millennia6. Modern muscle research probably begins in 1674-1682 with the microscopic observation of cross-striations and myofibrils in muscle fibers by Leeuwenhoek6. Almost a century later, Luigi Galvani observed that frog muscle contracts vigorously when its nerve is touched with scalpel during a spark discharge from a distant electric machine7-9. Contraction could also be produced by connecting the leg nerve to the muscle through a metal conductor. The details of the complex electrical signaling mechanism advocated by Galvani were eventually formulated by Hodgkin, Huxley and Katz in their famous equation10, 11 that became the foundation of electrophysiology. The remarkable observations of Ringer on the effects of extracellular Ca2+ on the contractility of frog heart and skeletal muscles12-15 represent the first major step in the recognition of Ca2+ as a key regulator of muscle contractility16, 17. From the 1980's to the present day a burst of discoveries in the muscle contractility field was realized due to the introduction of muscle contractility and fatigability protocols in murine skeletal muscles18. Jones and Edwards were the first to suggest that low frequency intermittent fatigue (exercise-induced reduction in force)19 was associated with changes in the ECC machinery and not the contractile apparatus. In the late 1980's and early 1990's, Kolkeck et al20, Kolbeck and Nosek 21, and Reid 22 were using diaphragm muscle from rodent models to study the effects of theophyllines, cortiosterone, and free radicals on skeletal muscle contractility, while Brooks and Faulkner were the first to report on measurements of repeated force and power measurements in fast- and slow-muscles from mice22. In addition, Lannegren, Westerblad, Lamb, and Westerblad were the first to directly link ex vivo contractility with intracellular Ca2+ regulation and started questioning the role of acidosis in muscle fatigue23, 24.
Our laboratories have significantly contributed since the early 2000's towards understanding of novel genes with modulatory and regulatory roles on muscle ECC with critical roles in muscle contractility, fatigability, and aging by using a combination of intact mouse muscle contractility studies, intracellular Ca2+ monitoring in intact and skinned muscle fibers and molecular-genetic manipulations3-5, 25-29.
Here we detailed the experimental protocol for measuring contractility of murine isolated soleus and extensor digitorum longus (EDL) muscles, which correspond to a mostly slow-oxidative (type I and IIa muscle fibers) and a mostly fast-glyocolytic muscle (type IIb and IIx muscle fibers) with distinct contractile properties. In this protocol, intact muscle-tendon complexes were isolated and bathed in an ADI PowerLab Radnotti chamber system supplied with either pure oxygen or a mixture of oxygen (95%) and CO2 (5%). Contractile forces were generated by electrical stimulations from a Grass stimulator and detected using a force transducer that was integrated with an ADI PowerLab/400 system, allowing customization of macro routines to control the acquisition, collection, digitization, and storage of data. This setup can measure muscle force, muscle power, as well as the force vs. frequency relationship, muscle fatigue, recovery from muscle fatigue, speed and overall kinetic properties of muscle contraction. In addition, the effects of drugs on muscle contraction can be monitored through these experiments.
Advantages of this method lay in removing the neuronal and vascular components away from the skeletal muscle, allowing direct assessment of the intrinsic properties of contracting muscle. In addition, ex vivo contractility assays allow manipulation of the extracellular milieu surrounding the isolated muscles, which enables the use of pharmacological manipulations of various ion permeation channels and transporters in order to define their physiological roles for skeletal muscle function.
This ex vivo system has allowed us to recently discover a distinct alternan behavior in certain mutant muscle preparations, which were linked to altered intracellular Ca2+ handling properties4. Alternans are defined as fluctuating burst episodes of contractile force during the decline phase of the fatiguing profile. During these events contractile forces momentarily increase above its previous level of force during fatiguing stimulation, perhaps because either more Ca2+ is being released or the contractile machinery has become more sensitive to Ca2+ 30. Treatment of cyclopiazonic acid (CPA), a reversible blocker of sarcoplasmic-endoplasmic reticulum calcium ATPase (SERCA), caffeine, an agonist of ryanodine channel (RyR) and repeated fatiguing stimulations can all induce mechanical alternans4, suggesting that alternans are directly related to modulation of the E-C coupling process. Demonstration of the method to induce and record mechanic alternans in in vitro contractility setup serves as an example to show the diversified experimental parameters that could be obtained with this system or similar ones, based on individual research interests.
This method may be of interest for researchers studying muscle physiology. Similar setup can also be used for isolated skeletal muscle-tendon/ligament complexes from other anatomical locations, as well as for single fibers and muscle strips.
Protokół
Solution composition:
2.5 mM Ca2+ Tyrode solution: 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 2.5 mM CaCl2, 2 mM MgCl2 and 10 mM glucose
0 mM Ca2+ Tyrode solution: 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 2 mM MgCl2, 0.1 mM ethylene glycol tetraacetic acid (EGTA) and 10 mM glucose
Note: bathing solution should be saturated with 100% O2 if using the above solution, but with 95% O2 with 5% CO2 if using bicarbonate-based buffers to keep pH constant. 2.5 mM Ca2+ is added to the bathing buffer to recapitulate the level of Ca2+ found in the extracellular space and 10 mM glucose is important since mitochondria are still functioning in these muscles to continuously produce ATP in the presence of glucose.
1. Setting-up the Ex Vivo Contractility Experiment Using the ADI PowerLab System
- A schematic drawing of a 4-channel ex vivo contractility system is shown in Figure 1. A computer controls a stimulator to generate square-wave pulses, which are filtered by the isolation unit to reach a pair of platinum wire surrounding each isolated muscle-tendon complex. This is also called field stimulation. Contraction of the muscle responding to the stimulation was sensed by a force transducer, the signal generated from which was amplified, filtered and transferred back to the computer by an A/D converter (signal conditioning). The signal is then digitized and can be saved for later analyses.
- To prepare for an ex vivo contractility experiment, first turn on the field stimulator, the A/D converter (in this case ADI Power Lab) or a similar software (e.g., LabView), followed by the computer.
- Open Chart4 software (or other software version that compatible with the system) and initiate a 4-channel task; proper communication of the software and the hardware is indicated by starting configuration of the hardware, and the START button on the software becomes operational (live); the 4-channel function allows simultaneous measurement of a pair of muscles from a wild type and a mutant mice, or 2 EDL and 2 soleus muscles from the same mouse, and it can be used for other muscles such as the diaphragm (whole diaphragm, hemi-diaphragm, or diaphragm muscle strips) and the tibialis anterior; it can also be used for cardiac muscle preparations and for bundles of muscles, particularly if one is using rat muscles. In this case the size of the muscles may limit oxygen diffusion so muscle bundles are thus a better choice. However, expert dissection techniques are required for muscle bundle preparations.
- Calibration of the force transducer: this is to ensure the comparability of dataset generated at different channels and at different time. First start recording, sequentially hang a 1-g, 2-g and 5-g weights on the sample grove of the force transducer, stop recording, calculate corresponding changes in mV shown in each channel of Chart4 software, plot the ΔmV and the weight to check linearity and determine the conversion factor between mV and grams of force. We recommend performing these calibrations either at the beginning or at the end of each experiment to assure that a specific calibration is obtained for each specific experiment.
- Check proper connection of the Teflon tube, drain any liquid present in the tissue bath tubing, wash the chambers three times or more with ddH2O and fill with 20~25 ml 2.5 mM Ca2+ Tyrode solution. Ca2+ and glucose are provided in the bathing solution to help maintain membrane integrity, optimal Ca2+ loading of the SR, and a readily available source of energy for generation of ATP by the muscle itself, since mitochondria remain fully functional in these preparations.
- Check proper connection of the oxygen supply, open the oxygen tank, and adjust oxygen flow to provide diffusive and homogeneous bubbling of the chamber. Oxygen levels can easily be determined as a function of contractile force vs. time. A flow meter can also be installed, particularly if researchers are interested in studying the effects of hypoxia or hyperoxia. If muscles become hypoxic, force naturally decreases. While hyperoxia can also damage muscles but its effects might be harder to be detected because most experiments are performed under artificial hyperoxic conditions to compensate for the absence of a normal blood supply to the muscles. In most systems it is possible to bubble the different chambers with the same amount of oxygen simply by controlling the flow of oxygen to each chamber constant.
- Adjust sensitivity of all channels to ensure the maximal force resolution without saturating the channel. Channel sensitivity can vary considerably as function and age of the animal, experimental temperature, genetic manipulations, muscle size, mouse strain, and the ability of experimenter to properly dissect muscles free of damages. In our experience, when measuring soleus contractile force, the sensitivity varies from 0.5 to 10 mV/cm, while in the case of EDL, the sensitivity could vary from 1-20 mV/cm.
- A protocol for the fatiguing stimulation needs to be established. In the Chart4 software a macro can be used. To program a new Macro: in the Chart4 software, click start measuring, then click Macro, Macro Command, start recording, begin repeat, choose desired stimulus frequency, and then end repeat. For an equilibration protocol, stimulus is repeated every minute for 30 min and for fatiguing protocol, stimulus is repeated every 2 sec for 5 min. These changes in the periodicity of the stimulation, directly affect the duty cycle, which is also a function of the stimulation duration. These parameters can be varied to test different aspects of contractility and fatigability.
2. Preparing Intact Muscle Bundles
- EDL dissection: mouse is sacrificed following NIH guidelines and IACUC institutional animal protocols. Mouse is sacrificed by cervical dislocation. Mouse is then arranged at the lateral position. EDL muscle is a fast-glycolytic muscle with pale pink-white color. It is about 10-13 mm long and weighs 8-11 mg in wild type C57BL/6 mouse. The EDL has the functions of extending toes 2-5, and dorso-flexing the foot at the ankle. It is innervated by the peroneal nerve. To dissect the EDL, make a superficial skin incision and cut open the fascia between the anterior tibialis and the posterior muscle group, and localize the origin (proximal) where ligament is connected to the lateral condyle of tibia and superior 3/4 of the anterior surface of fibula (interosseous margin). Cut the ligament with delicate ophthalmic scissors as distally as possible from the muscle; this procedure releases the proximal origin of the EDL muscle. Hold the ligament with a blunt forceps and pull slowly to free up the EDL. It might be necessary to cut some of the perimysium surrounding the EDL and other muscles around the EDL, a step that is critical since damage can occur to the EDL muscle during this delicate step. Next, move to the insertion region where the four distal tendons insert into the middle and distal phalanges of digits 2-5. Cut the tendons as far as possible from the muscles.
- Transfer the isolated EDL muscle into a dissection dish containing isotonic Tyrode solution. Some mutant, transgenic, and knockout animal models have very fragile muscles and the utilization of Ca2+-free Tyrode solution is necessary to prevent Ca2+-induced muscle damage before contractility measurements. Next, use a surgical knot to tie tightly at both ends of the EDL muscle as distally from the muscle as possible. A good measure is to tie slightly above the mid-point lengthwise of the ligament or the tendon. Use 6-0 size suture for this procedure, and transfer the EDL muscle to the O2-saturated tissue bath chamber, mount the muscle on the sample groove of the force transducer and the stationery hook on the bottom of the bath chamber, repeat the process for the other leg. We are also beginning to utilize a new method that holds the muscles with clamps instead of sutures. If the major goal is to study kinetic properties and/or to obtain muscle power, it is recommended that suture be as short as possible or the suture be replaced with a metal rod.
- Soleus dissection: the soleus is a mostly slow-oxidative muscle with rich red color. It is ~1 mm shorter than the EDL but weighs slightly more than the EDL. It is innervated by the tibial nerve and it performs the action of plantar flexing the foot. To dissect the soleus, access the posterior lateral side of the leg, push aside the gastrocnemius muscle that normally covers the soleus, and identify the muscle with dark red color. At the origin (proximal), cut the ligaments connecting to the proximal half of posterior tibia along the soleal line and proximal 1/3 of the posterior fibula; next, at the insertion (distal), cut the calcaneal tendon that inserts into the posterior calcaneus. Carefully free up the soleus and properly mount the soleus muscle in the bath chamber as for the EDL.
3. Measuring Contractility of the Isolated Skeletal Muscles
- Once the muscles are mounted in individual tissue bath chambers, start recording. Most similar systems allow zeroing of the baseline of force recording. This function is usually associated with the amplifier, in the specific case of the PowerLab system, as function of the bridge amplifier. It facilitates observation of baseline changes and provides a convenient way for all muscle contractions to be analyzed from zero. Isolated muscles are then stimulated with squared-wave pulses that can range widely as function of the chamber size, platinum wires thickness, distance between the wires, and even the composition of the experimental solution. We have employed current of 60 mA (we have noted that currents above 350 mA seem to be detrimental to muscle preparations) and stimulatory trains of 350, 500, and 1000 ms, depending on the goals of a specific protocol. The individual square wave pulses should have duration ranging from 0.3-1 ms.
- The next step is to select a frequency of stimulation capable of producing a fused tetanic stimulation (ex: ~100 Hz to allow production of maximal force in EDL muscle and ~60 Hz in soleus muscle) while slowly and carefully stretching the muscles to identify the optimal length of these muscles. As muscles are carefully stretched, wait for 30 s and stimulate at 100 Hz; wait 30 s and stretch again, and then repeat the stimulation, until the point where the force does not increase anymore.
- These muscles have undergone significant changes in environment. It is recommended that muscles be allowed to adapt to the new environment, a step in our protocols termed "equilibration". Equilibrate these muscles at the same frequency of stimulation used during the stretching phase, 100 Hz for 20-30 min, until at least 5 consecutive tetanic contractions are completely stable (not reducing, not increasing, stable baseline). Periodicity of the stimulatory trains (100 Hz, 500 ms duration, 1 ms individual pulse) during equilibration is 1 min, which equals to a duty cycle of 1.66%, a non-fatiguing stimulation. In this context, a duty cycle of 1.66% means that over a total of 100%, muscles are working 1.66% of the time, by increasing the duty cycle muscles can eventually be induced to fatigue. If otherwise muscles of healthy, wild type mouse show a fatiguing profile during this equilibration period, it is possible that they were damaged during dissection, hypoxia is occurring in the chambers/muscles, or excessive electrolysis through the stimulating electrodes is generating free radicals. Westerblad has previously suggested that currents larger than 400 mA induce the formation of free radicals23. Obviously, the highest quality platinum is suggested, since other metals will certainly lead to free radical formation and muscle toxicity.
- Obtain the force vs. frequency relationship (FF) by stimulating the muscles with the following stimulation frequencies: 1-140 Hz (in increments of 5-10 Hz), with a periodicity of 30-60 s when performing experiments at 25 °C. When performing experiments at 37 °C, extend the FF to higher frequencies of up to 300 Hz for diaphragm muscle, 180-200 Hz for soleus, and 220-250 Hz for EDL. Next, identify the frequency of stimulation that generates maximal tetanic force (Tmax) and approximately ½ maximal tetanic force (1/2 Tmax); sometimes it is difficult to obtain the exact ½ Tmax for both EDL and soleus muscles if only one stimulator source is being used, because of the intrinsic differences between these muscles. A viable solution is to identify a frequency that produces 30-70% of the Tmax. The rationale for these frequencies of stimulation is that Tmax provides important information of contractile machinery events/modulation, while the ½ Tmax provides information more pertinent to the Ca2+ regulation and the ECC process. To be certain that maximal force has been achieved, 10-20 mM caffeine can be added to the bathing solution while stimulating the muscle. If maximal force has been reached, force will not increase in the presence of caffeine. The FF is shifted to the right and forces tend to be slightly higher at higher experimental temperatures. Using this system, any other stimulation frequencies can be performed if desirable. The distinct contractile profile of a fast-glycolytic EDL muscle and a slow-oxidative soleus muscle is shown in Figure 2.
- After equilibration, fatigue the muscles at ½ Tmax for 5 min, with stimulation interval of 2 s and 25% duty cycle (Figure 3). This specific fatiguing protocol is believed to better reflect the contribution of the sarcoplasmic reticulum Ca2+ release for muscle contractility31.
- Recover the muscle at ½ Tmax for 30 min or until the force is stable, at 1 min interval. An additional fatiguing protocol can be performed now using Tmax stimulation, which is believed to reflect the status of the contractile machinery31. Another option is to extend the fatiguing protocol to intercalate stimulatory trains that generate Tmax and ½ Tmax during fatigue and to recover the muscles with the same type of stimulation.
- Repeat the FF as described in step 3.4; the rationale being that phenotypic differences between different strains, disease models, or drug treatments can be observed by analyzing the FF before and after fatigue. We have for example previously reported that after fatigue the FF of young wild type muscles is shifted to the left, while in muscles from aged muscles, it is shifted to the right, suggesting differential modulatory effects of skeletal muscle fatigue as a function of aging.
- To probe the contribution of the extracellular Ca2+ entry in muscle contractility, bathing solution can be changed into a solution containing no Ca2+ but 0.1 mM EGTA (0 mM Ca2+ Tyrode solution)32. Alternatively, different blockers of store-operated channel can be applied in the bathing solution. A few examples are: 2-aminoethyl diphenylborinate (2-APB), SKF96365, 3,5-bis(trifluoromethyl)pyrazole 2 (BTP-2) and azumolene, etc.33, 34. Caffeine can be used to probe the function of the ryanodine receptor, and KCl can be used to assess the overall depolarization properties of these preparations. Other drugs can also be used to investigate the important modulation of force during fatigue by the Na+, K+, and the Na+-K+ pumps35. Most of these drugs have relatively small sizes and seem to quickly diffuse into these muscle preparations as evidenced by their immediate effects. The lack of an effect by any given drugs does not necessarily mean that the drug is ineffective and additional tests using dose much higher than that used in single muscle fiber experiments are sometimes necessary.
- One unique application of this ex vivo system led to the recent discovery of mechanic alternans in tric-a-/- muscles4, 30. Alternans are defined as fluctuating burst episodes of contractile force during the decline phase of the fatiguing profile. During these events contractile force can momentarily increase above its previous level of force during fatiguing stimulation because that either more Ca2+ is being released or the contractile machinery has become more sensitive to Ca2+. The contractile force outbreak has to be 50% higher than its previous force and the outbreaks should be seen at least 10 times during the 5-min fatigue stimulation process. Mechanic alternans are not common in skeletal muscle from the wild type mice, but can be seen in some mutant muscles with perturbation of the intracellular Ca2+ signaling process such as the Trimeric Intracellular Cation Channel type A (tric-a)-/- muscle4. Mechanic alternans can be induced through fatiguing stimulations, treatment with caffeine and cyclopiazonic acid (CPA), see Figure 3 for a representative recording of these mechanical alternans. The nature of this phenomenon is quite intriguing, whereas a muscle that is fatiguing seems able to momentarily produce more force. In our previous publication, combination with single cell Ca2+ analysis reveals that appearance of alternans is a result of SR Ca2+ overload and unstable SR. We believe that a deeper understanding of alternans might lead to a better understanding of the ECC process.
- At the end of the experiment, measure the length and weight of individual muscles using calibrated caliper and analytical balance, flash frozen the muscle in liquid nitrogen and save at -80 °C, since biochemical analyses can be performed in these muscles. These muscles can also be mechanically-skinned for detailed probing of the ECC process, or be chemically-skinned for determination of essential contractile properties in the absence of ECC regulatory mechanisms.
- Recorded muscle force (mV) is first converted to gram force based on calibration results and then normalized to the physiological cross-sectional area (PCSA) using the following formula: muscle force (N/cm2) = (force (g) x muscle length (cm) x 1.06)/(muscle weight (g) x 0.00981)5,36. Alternatively, muscle force can be normalized to total protein or total actin content of the individual muscle using Bradford protein assay/Commossie blue staining quantification. Under certain conditions whereas muscle mass could be severely affected due to diseases, senescence, drug treatments, force normalization based on muscle mass, protein and/or actin content can provide a more stable readout.
4. Representative Results
Typical room temperature contractile forces of EDL and soleus responding to low, intermediate and high frequency stimuli are shown in Figure 2. The EDL contraction induced by 5 Hz stimulus remains as individual twitches due to the fast action of the SERCA Ca2+ ATPase and the intrinsic Ca2+ sensitivity properties of contractile machinery, while the soleus contraction by 5 Hz starts to fuse (slower ATPase and higher sensitivity to Ca2+ of the contractile machinery), but the peak forces are still separate. At 20 Hz stimulation, EDL contractions are partially fused while that of the soleus forms a completely fused tetanic force. At the frequency of stimulation that produces Tmax stimulation, which can vary at room temperature from 80-110 Hz for EDL and 60-90 Hz for soleus, fast upstroke and fast relaxation of the tetanic force in EDL are noted, which is contrary to the slow characteristics of the soleus muscle. Figure 2B demonstrates that the force-frequency curve of the EDL muscle is shifted to the right as compared to the soleus muscle, indicating that soleus muscles are more sensitive to Ca2+ release at any given frequency of stimulation due to the presence of the slow myosin and troponin isoforms. In addition, the contractile machinery responds with relative more force in soleus muscle at lower frequencies. Figure 3 shows a normal fatigue profile of the EDL (upper panel) and soleus muscle (middle panel). Note the faster decline in contractile force under fatiguing stimulation in the EDL muscle and the higher decrease in force at the end of the 5-min fatigue protocol. Finally, a typical mechanical alternan profile of a mutant muscle was shown in Figure 3 (lower panel), which was defined as momentary force outbreaks during the declining phase of muscle fatigue profile. The contractile force outbreak has to be 50% higher than its previous force and the outbreaks should be seen at least 10 times during the 5-min fatigue stimulation process.
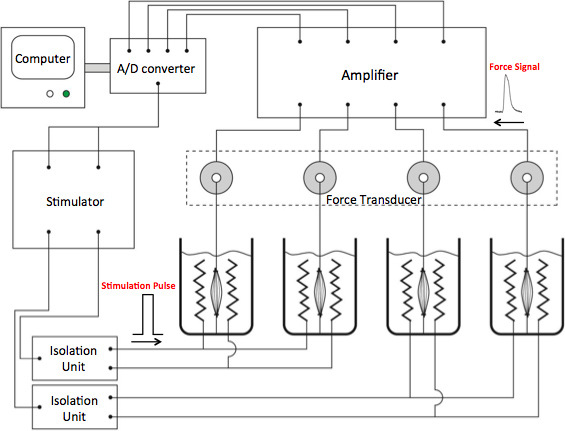
Figure 1. Schematic drawing of a 4-channel ex vivo contractility system. Square-wave pulses are generated by a computer controlling a Grass stimulator. Two stimulation isolation units filter the stimulus originating from the electrical stimulator unit to remove any fluctuations in the electrical signal and to establish a stable square wave signal. This filtered electrical signal is sent to the 4 bathing chambers containing the platinum wire electrodes surrounding each isolated muscle. Ultimately, it is the current across the two electrodes (called field stimulation) that generate an action potential, inducing the contraction of the muscle. This contraction is detected by a specific force transducers, transmitted to the bridge amplifiers, filtered, averaged (signal conditioning) and recorded by computer software through an A/D converter.
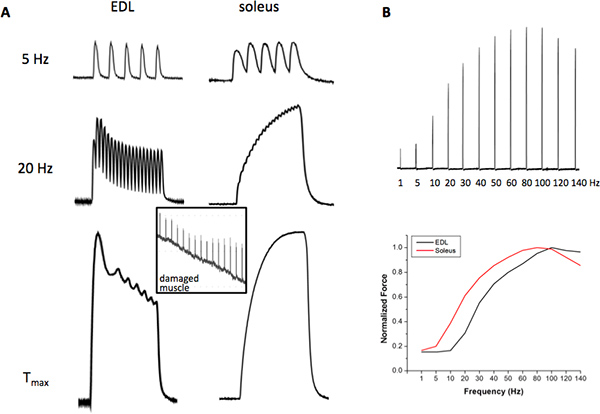
Figure 2. Representative contractile forces of EDL and soleus. (A) Contractile forces induced by 5 Hz (upper panel), 20 Hz (middle panel) and maximal tetanic force (Tmax) (lower panel); inlet shows a trace of contractile force of a damaged muscle; (B) a representative set showing the individual contractions of a force vs. frequency relationship in EDL (FF, upper panel) and the plotted curve resulting from the FF (lower panel). Click here to view larger figure.
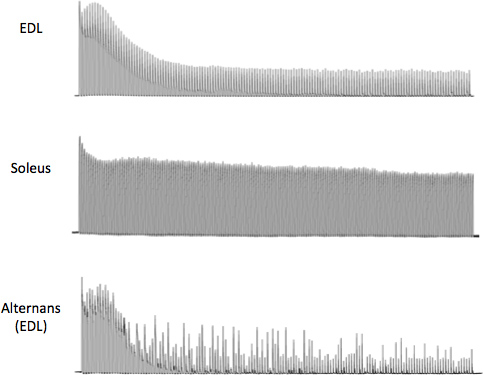
Figure 3. Representative fatiguing profile and mechanical alternans. A typical fast decline fatiguing profile of the EDL muscle (upper panel) and the slow decline fatiguing profile of the soleus muscle (middle panel). Fatiguing stimulation leads to the appearance of mechanical alternans in a tric-a-/- muscle with disturbed Ca2+ handling properties (lower panel).
Dyskusje
Measurement of contractile force and fatigability is important for the overall evaluation of skeletal muscle function. The major purpose of this assay is to identify changes in muscle force and fatiguing properties under certain pathological conditions, such as sarcopenia and muscle fatigue, and to test the effect of drugs/reagents on muscle contractility. Since the muscle force is closely related with intracellular Ca2+ release, extracellular Ca2+ entry and the crosstalk between these two, we can a...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported by AHA SDG 10SDG2630086 to Zhao X, RO1-AR061385 to Ma J and GO Grant RC2AR05896 to Brotto M.
Materiały
| Name | Company | Catalog Number | Comments |
| 2-APB | Tocris | 1224 | Blocker of a number of Ca2+ entry channels including SOC and TRP etc. |
| SKF96365 | Sigma | SKF-96365 | Blocker of a number of Ca2+ entry channels including SOC and receptor-mediated Ca2+ entry etc. |
| BTP-2 | Millipore | 203890-5MG | Relatively specific SOC blocker |
| CPA | Sigma | C1530 | Reversible SERCA blocker |
| caffeine | Sigma | C0750 | Fast action RyR agonist |
| Radnoti Four Unit Tissue Organ Bath System | Radnoti | 159920 | |
| Combination Tissue Support/Stimulating Electrode | Radnoti | 160151 | Vertical Zig Zag Type with tissue support |
| Quad Bridge Amp | ADInstruments | FE224 | |
| PowerLab/400 | ADInstruments | This product is no longer available. Choose other version of the data acquisition system. | |
| Force Transducers (5 mg - 25 g) | ADInstruments | MLT0201/RAD | |
| Chart v4.02 | ADInstruments | LabChart 7.3 is the latest version of Chart software. | |
| S8800 Dual Pulse Digital Stimulator | GRASS TECHNOLOGIES | This product is no longer available. S88X Dual Output Square Pulse Stimulator is a newer stimulator. | |
| RF Transformer Isolation Unit | GRASS TECHNOLOGIES | Model SIU5 |
Odniesienia
- Winegrad, S. Role of intracellular calcium movements in excitation-contraction coupling in skeletal muscle. Fed. 24, 1146-1152 (1965).
- Sandow, A. Excitation-contraction coupling in skeletal muscle. Pharmacol. Rev. 17, 265-320 (1965).
- Thornton, A. M. Store-operated Ca(2+) entry (SOCE) contributes to normal skeletal muscle contractility in young but not in aged skeletal muscle. Aging. 3, 621-634 (2011).
- Zhao, X. Ca2+ overload and sarcoplasmic reticulum instability in tric-a null skeletal muscle. J. Biol. Chem. 285, 37370-37376 (2010).
- Brotto, M. A. Defective maintenance of intracellular Ca2+ homeostasis is linked to increased muscle fatigability in the MG29 null mice. Cell Res. 14, 373-378 (2004).
- Florkin, M. Machina carnis. The Biochemistry of Muscular Contraction in its Historical Development. Med. Hist. 17, 316-317 (1973).
- Galvani, A., Aldini, J. De viribus electricitatis in motu musculari commentarius. ApudSocietatem Typographicam. , (1792).
- Fulton, J. F., Fulton, J. F., Wilson, L. G. . Selected Reading in the History of Physiology. , (1930).
- Piccolino, M. Luigi Galvani and animal electricity: two centuries after the foundation of electrophysiology. Trends Neurosci. 20, 443-448 (1997).
- Hodgkin, A. L. The Croonian Lecture: Ionic Movements and Electrical Activity in Giant Nerve Fibres. Proceedings of the Royal Society of London. Series B, Biological Sciences. 148, 1-37 (1958).
- Hodgkin, A. L. . The Sherrington Lectures VII the Conduction of the Nervous Impulse. , 71964 (1965).
- Ringer, S. A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J. Physiol. 4, 29-42.3 .
- Ringer, S. Further experiments regarding the influence of small quantities of lime, and other salts on muscular tissue. J. Physiol. 7, 291-308 .
- Ringer, S., Buxton, D. W. Concerning the action of calcium, potassium and sodium salts upon the eel's heart and upon the skeletal muscles of the frog. J. Physiol. 8, 15-19 .
- Ringer, S. Regarding the action of lime, potassium and sodium salts on skeletal muscle. J. Physiol. 8, 20-24 (1887).
- Campbell, A. K. . Intracellular Calcium its Universal Role as Regulator. , (1983).
- Mol, J. . Cell Cardiol. 16, ll3-ll6 (1984).
- Ridings, J. W., Barry, S. R., Faulkner, J. A. Aminophylline enhances contractility of frog skeletal muscle: an effect dependent on extracellular calcium. J. Appl. Physiol. 67, 671-676 (1989).
- Fitts, R. H. The cross-bridge cycle and skeletal muscle fatigue. J. Appl. Physiol. 104, 551-558 (2008).
- Kolbeck, R. C., Speir, W. A. Diaphragm contactility as related to cellular calcium metabolism: Influence of theophylline and fatigue. American Review of Respiratory Disease. 139, 495 (1989).
- Kolbeck, R. C., Nosek, T. M. Fatigue of rapid and slow onset in isolated perfused rat and mouse diaphragms. J. Appl. Physiol. 77, 1991-1998 (1994).
- Moore, B. J. Diaphragm atrophy and weakness in cortisone-treated rats. J. Appl. Physiol. 67, 2420-2426 (1989).
- Lannergren, J., Westerblad, H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J. Physiol. 434, 307-322 (1991).
- Westerblad, H. Spatial gradients of intracellular calcium in skeletal muscle during fatigue. Pflugers Arch. 415, 734-740 (1990).
- Zhao, X. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol. Genomics. 23, 72-78 (2005).
- Wang, X. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ. Res. 107, 76-83 (2010).
- Cai, C. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 11, 56-64 (2009).
- Shen, J. Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca(2+) homeostasis. Nat. Cell Biol. 11, 769-776 (2009).
- Romero-Suarez, S. Muscle-specific inositide phosphatase (MIP/MTMR14) is reduced with age and its loss accelerates skeletal muscle aging process by altering calcium homeostasis. Aging (Albany NY). 2, 504-513 (2010).
- Yazawa, M. TRIC channels are essential for Ca2+ handling in intracellular stores. Nature. 448, 78-82 (2007).
- Brotto, M. A., Nosek, T. M., Kolbeck, R. C. Influence of ageing on the fatigability of isolated mouse skeletal muscles from mature and aged mice. Exp. Physiol. 87, 77-82 (2002).
- Zhao, X. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell. 7, 561-568 (2008).
- Pan, Z. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat. Cell Biol. 4, 379-383 (2002).
- Zhao, X. Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. J. Biol. Chem. 281, 33477-33486 (2006).
- Renaud, J. M. Modulation of force development by Na+, K+, Na+ K+ pump and KATP channel during muscular activity. Can. J. Appl. Physiol. 27, 296-315 (2002).
- Brotto, M. A. Functional and biochemical modifications in skeletal muscles from malarial mice. Exp. Physiol. 90, 417-425 (2005).
- Brotto, M. A. Hypoxia and fatigue-induced modification of function and proteins in intact and skinned murine diaphragm muscle. Pflugers Arch. 440, 727-734 (2000).
- Smith, M. A., Reid, M. B. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 151, 229-241 (2006).
- Bagni, M. A., Cecchi, G., Colomo, F. Myofilament spacing and force generation in intact frog muscle fibres. J. Physiol. 430, 61-75 (1990).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone