Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Fiber-optic Implantation for Chronic Optogenetic Stimulation of Brain Tissue
W tym Artykule
Podsumowanie
The development of optogenetics now provides the means to precisely stimulate genetically defined neurons and circuits, both in vitro and in vivo. Here we describe the assembly and implantation of a fiber optic for chronic photostimulation of brain tissue.
Streszczenie
Elucidating patterns of neuronal connectivity has been a challenge for both clinical and basic neuroscience. Electrophysiology has been the gold standard for analyzing patterns of synaptic connectivity, but paired electrophysiological recordings can be both cumbersome and experimentally limiting. The development of optogenetics has introduced an elegant method to stimulate neurons and circuits, both in vitro1 and in vivo2,3. By exploiting cell-type specific promoter activity to drive opsin expression in discrete neuronal populations, one can precisely stimulate genetically defined neuronal subtypes in distinct circuits4-6. Well described methods to stimulate neurons, including electrical stimulation and/or pharmacological manipulations, are often cell-type indiscriminate, invasive, and can damage surrounding tissues. These limitations could alter normal synaptic function and/or circuit behavior. In addition, due to the nature of the manipulation, the current methods are often acute and terminal. Optogenetics affords the ability to stimulate neurons in a relatively innocuous manner, and in genetically targeted neurons. The majority of studies involving in vivo optogenetics currently use a optical fiber guided through an implanted cannula6,7; however, limitations of this method include damaged brain tissue with repeated insertion of an optical fiber, and potential breakage of the fiber inside the cannula. Given the burgeoning field of optogenetics, a more reliable method of chronic stimulation is necessary to facilitate long-term studies with minimal collateral tissue damage. Here we provide our modified protocol as a video article to complement the method effectively and elegantly described in Sparta et al.8 for the fabrication of a fiber optic implant and its permanent fixation onto the cranium of anesthetized mice, as well as the assembly of the fiber optic coupler connecting the implant to a light source. The implant, connected with optical fibers to a solid-state laser, allows for an efficient method to chronically photostimulate functional neuronal circuitry with less tissue damage9 using small, detachable, tethers. Permanent fixation of the fiber optic implants provides consistent, long-term in vivo optogenetic studies of neuronal circuits in awake, behaving mice10 with minimal tissue damage.
Protokół
*All materials along with respective manufacturers and/or vendors are listed below the protocol.
1. Assembly of Implant
- Prepare a mixture of heat-curable fiber optic epoxy by adding 100 mg of hardener to 1 g of resin.
- Measure and cut approximately 35 mm of 125 μm fiber optic with 100 μm core by scoring it with a wedge-tip carbide scribe. Position the scribe perpendicular to the fiber optic and score in a single, unidirectional motion. Cutting the fiber completely will damage the fiber core.
- Insert a LC ceramic ferrule with a 127 μm bore into the vice, convex side pointed down.
- Insert the fiber optic into the ferrule. The fiber optic should slide in smoothly and marginally protrude beyond the convex end of the ferrule (Figure 1a).
- Apply one drop of heat-curable fiber optic epoxy to the flat end and heat with heat gun until epoxy turns black. The epoxy should fill the ferrule as it is heated and before curing. The epoxy should cure within ~1 min of constant heat application.
- Clean off any epoxy along the sides of the ferrule, as it will obstruct interfacing with the coupler.
- Polish the convex end of the ferrule using a LC fiber optic polishing disc (FOPD) on aluminum oxide polishing sheets on the polishing pad (Figure 1b). Make circular rotation patterns and polish on four grades in the following order: 5, 3, 1 0.3 μm grit.
- Cut the fiber optic at the flat end to the appropriate length such that it targets the region of interest. The length can be determined using the stereotaxic atlas.
- Test the implant by connecting it to the laser via the coupler cord described below. The polished end of the implant is inserted into sleeve of the coupler and should make direct contact with the opposing ferrule. The implant should be able to maintain 10 mW of light output, measured at the tip of the implant fiber. A bad implant will have a weak focal point near the tip of the fiber optic.
- Store the finished implants (Figure 1c) in foam until use.
2. Assembly of Fiber Optic Coupler Cord
- Prepare a mixture of heat-curable fiber optic epoxy as above.
- Measure and cut an appropriate length of 220 μm fiber optic with 200 μm core by scoring it with a wedge-tip carbide scribe. The length of the fiber should allow the mouse to move freely around the housing but not allow the mouse to chew through the fiber.
- Insert the fiber optic into a length of furcation tubing slightly longer than the fiber optic length. The tubing should have an inner diameter slightly larger than the fiber optic.
- Strip ~25mm at one end of the fiber optic and insert it into the metal end of a Multimode FC MM Ferrule Assembly with 230 μm bore until it stops. The fiber optic should stick out through the ferrule end (Figure 2a).
- Secure the connection with cyanoacrylate (super glue) at the metal end. Cover the connection with a Connector Boot and polish the ferrule end with a FC FOPD. Make circular rotation patterns and polish on four grades in the following order: 5, 3, 1, 0.3 μm grit (Figure 2b).
- Strip and insert the other end of the fiber optic into a LC ceramic ferrule (230 μm i.d. bore) with the convex end distal. Apply a drop of epoxy to the flat end and heat until cured.
- Polish the convex end of the ferrule using a FC FOPD on aluminum oxide polishing sheets as described above.
- Slide a LC ferrule sleeve over the convex end of the ferrule until the midpoint of the sleeve.
- Place heat-shrink tubing over the furcation tubing and sleeve and heat to secure and protect the connection (Figure 2c).
- Test the coupler by connecting it to the laser source and measuring the light output through the coupler with a spectrophotometer. The light loss between the laser output and the measured coupler output should not exceed 30%.
3. Surgical Implantation
*This is a tips-only procedure. Instruments are sterile but gloves do not need to be due to constant manipulation between the instruments and equipment.
- Anesthetize the mouse with an intraperitoneal injection Ketamine/Xylazine mixture 100 and 10 mg/kg respectively using a 30-gauge needle.
- Shave the scalp with clippers. Wipe the scalp with 70% isopropyl alcohol followed by Betasept wipe (4% Chlorhexidine solution) for two min, repeating once.
- Place the mouse in the sterotaxic rig and secure the head, ensuring that the skull is level. Apply ophthalmic ointment to eyes to prevent dryness and postoperative pain. Maintain anesthesia using volatized isoflurane (1-3% diluted with oxygen depending on the physiological state of the mouse, which should be continuously monitored by response to a tail pinch).
- Make an incision through the midline of the scalp, exposing the cranium from the eye orbits to lambda. Push aside connective tissue as necessary.
- Use Serafin clamps to hold back the skin and maintain an access to the cranium (Figure 3a).
- Etch a checkered pattern throughout the surface of the cranium with a dental pick. Wash debris away with sterile saline. Dry thoroughly.
- Apply hydrogen peroxide (3%) to the exposed cranium with a cotton swab for ~2-3 sec to create micropores. Wash multiple times and dry thoroughly. Alternatively, anchors can be screwed into the cranium, as described in Sparta et al. (2012).
- Again, etch a checkered pattern throughout the cranium with a dental pick and wash away debris with saline. Dry thoroughly.
- Using a rotary tool, make a small burr hole craniotomy (<1 mm in diameter) with a sterile drill bit (autoclaved) above the region of interest, determined by the stereotaxic atlas calibrated to bregma and lambda. Be careful not to break the dura or damage any tissue. Wash away debris and dry thoroughly.
- Insert the fiber optic ferrule (implant) into the probe holder and connect to the stereotaxic arm.
- Position the implant in place directly above the region of interest using the stereotaxic arm (Figure 3b). If inserting the optical fiber in the brain tissue, the fiber should be advanced slowly at a rate of ~2 mm/min. The ferrule should rest on the remaining cranium.
- Prepare a mixture of dental cement. The mixture should have a low enough viscosity to easily apply across the cranium. Mixture will be usable for 2-4 min.
- Using a sterile toothpick, apply a thin, even layer of dental cement across the cranium and onto the lower portion of the implant. The base layer of dental cement should cover as much surface area on the cranium as possible. Do not let the dental cement come into contact with the skin of the mouse. This will lead to increased difficulty in suturing as well as irritation to the mouse. If the dental cement does come into contact with the skin, allow it to partially dry such that the entire layer of cement can be peeled off before setting.
- Allow it to dry completely.
- Apply even layers of dental cement to form a small mound over the cranium and around the implant, allowing each layer to dry completely (Figure 3c). Leave ~3-5 mm of the convex end of the ferrule clean of cement to allow for a smooth, unobstructed connection.
- Suture the scalp over the mound of dental cement and around the implant using sterile, single-use silk braided sutures (6-0) with a C22 needle. Remove after 7 days. Optional: Use Vet Bond for additional binding after suturing. Be sure to use minimal Vet Bond. Excess can lead to severe skin damage due to scratching.
- Immediately after surgery, mouse should be subcutaneously injected with Ketprophen (5 mg/kg) to decrease postoperative pain. This should be repeated 24 hr later. Apply topical analgesics (Bupivicaine) and antibiotics (Neosporin) to the sutured skin and around the base of the implant.
- Place the mouse in a cage over a heating blanket for recovery from anesthesia. Place the mouse in a sterile, postoperative recovery cage. The recovery cage should not contain any bedding in order to maintain temperature and avoid asphyxiation. The mouse can be returned to the original cage or a new cage once awake.
Proper assembly of the fiber optic implant and coupler results in minimal photon loss between the light source and the end of the fiber optic in the region of interest. Well-polished fiber optics should transmit light in a uniform, concentric circle (Figure 2d). With careful implantation and suturing, the implant causes no visible irritation to the mouse and can remain in place for long-term studies (Figure 3d, >1 month, unpublished observations) without any significant degradation of the fiber optic or the amount of light transmitted. Improper implantation or suturing can cause irritation and can result in the mouse scratching through its scalp, exposing the dental cement, or breakage of the ferrule from the dental cement due to persistent manipulation. A schematic diagram of the assembled system can be seen in Figure 4.

Figure 1. Assembly of implantable fiber optics. (a) The fiber optic is inserted into the ferrule, marginally protruding beyond the convex end indicated by the arrowhead. (b) The convex end of the ferrule is polished using a FOPD on progressively finer grades of polishing sheets. (c) The finished implantable fiber optic. Click here to view larger figure.

Figure 2. Assembly of fiber optic coupler used to tether the fiber optic rotary joint to the implant. (a) Fiber optic sticking through the ferrule assembly. (b) The ferrule side of the assembly is inserted into the FOPD and polished using progressively finer grades of polishing paper. (c) The ferrule sleeve is fitted over the ferrule and secured with heat shrink tubing. (d) The finished fiber optic coupler should produce a concentric light with minimal photon loss.

Figure 3. Surgical implantation the fiber optics. (a) The entire surface of the cranium is exposed and connective tissue is cleared. (b) The fiber optic implant is held in position with the stereotaxic arm. (c) Dental cement is applied fixing the fiber optic implant to the cranium. (d) >1 month after implantation, the skin has healed around the implant and there are no signs of irritation.
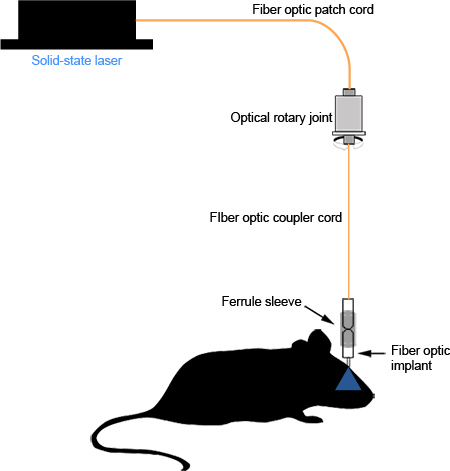
Figure 4. Schematic diagram of the functional system
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Optogenetics is a powerful new technique that allows unprecedented control over specific neuronal subtypes. This can be exploited to modulate neural circuits with anatomic and temporal precision, while avoiding the cell-type indiscriminate and invasive effects of electrical stimulation through an electrode. Implantation of fiber optics allows for consistent, chronic stimulation of neural circuits over multiple sessions in awake, behaving mice with minimal damage to tissue. This system, originally pioneered by Sparta ...
Access restricted. Please log in or start a trial to view this content.
Podziękowania
We would like to acknowledge that this technique was originally described by Sparta et al., 2012 and has been easily adapted for use in our lab.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| LC Ferrule Sleeve | Precision Fiber Products (PFP) | SM-CS125S | 1.25 mm ID |
| FC MM Pre-Assembled Connector | PFP | MM-CON2004-2300 | 230 μm Ferrule |
| FC MM Pre-Assembled Connector | PFP | MM-CON2004-2300 | 230 μm Ferrule |
| Miller FOPD-LC Disc | PFP | M1-80754 | For LC ferrules |
| Furcation tubing | PFP | FF9-250 | 900 μm o.d., 250 μm i.d. |
| MM LC Stick Ferrule 1.25 mm | PFP | MM-FER2007C-1270 | 127 μm ID Bore |
| MM LC Stick Ferrule 1.25 mm | PFP | MM-FER2007C-2300 | 230 μm ID Bore |
| Heat-curable epoxy, hardener and resin | PFP | ET-353ND-16OZ | |
| FC/PC and SC/PC Connector Polishing Disk | ThorLabs | D50-FC | For FC ferrules |
| Digital optical power and Energy Meter | ThorLabs | PM100D | Spectrophotometer |
| Polishing Pad | ThorLabs | NRS913 | 9" x 13" 50 Durometer |
| Aluminum oxide Lapping (Polishing) Sheets: 0.3, 1, 3, 5 μm grits | ThorLabs | LFG03P, LFG1P, LFG3P, LFG5P | |
| Standard Hard Cladding Multimode Fiber | ThorLabs | BFL37-200 | Low OH, 200 μm Core, 0.37 NA |
| Fiber Stripping Tool | ThorLabs | T10S13 | Clad/Coat: 200 μm / 300 μm |
| SILICA/SILICA Optical Fiber | Polymicro Technologies | FVP100110125 | High -OH, UV Enhanced, 0.22 NA |
| 1x1 Fiberoptic Rotary Joint | doric lenses | FRJ_FC-FC | |
| Mono Fiberoptic Patchcord | doric lenses | MFP_200/230/900-0.37_2m_FC-FC | |
| Heat shrink tubing, 1/8 inch | Allied Electronics | 689-0267 | |
| Heat gun | Allied Electronics | 972-6966 | 250 W; 750-800 °F |
| Cotton tipped applicators | Puritan Medical Products Company | 806-WC | |
| VetBond tissue adhesive | Fischer Scientific | 19-027136 | |
| Flash denture base acrylic | Yates Motloid | ColdPourPowder+Liq | |
| BONN Miniature Iris Scissors | Integra Miltex | 18-1392 | 3-1/2"(8.9cm), straight, 15 mm blades |
| Johns Hopkins Bulldog Clamp | Integra Miltex | 7-290 | 1-1/2"(3.8 cm), curved |
| MEGA-Torque Electric Lab Motor | Vector | EL-S | |
| Panther Burs-Ball #1 | Clarkson Laboratory | 77.1006 | |
| Violet Blue Laser System | CrystaLaser | CK473-050-O | Wavelength: 473 nm |
| Laser Power Supply | CrystaLaser | CL-2005 | |
| Dumont #2 Laminectomy Forceps | Fine Science Tools | 11223-20 | |
| Probe | Fine Science Tools | 10140-02 | |
| 5"Straight Hemostat | Excelta | 35-PH | |
| Vise with weighted base | Altex Electronics | PAN381 |
Odniesienia
- Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neuronal activity. Nat Neurosci. 8, 1263-1268 (2005).
- Arenkiel, B. R. In Vivo Light-Induced Activation of Neural Circuitry in Trangenic Mice Expressing Channelrhodopsin-2. Neuron. 54, 205-218 (2007).
- Gradinaru, V. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 141, 165-16 (2010).
- Luo, L., Callaway, E. M., Svoboda, K. Genetic dissection of neural circuits. Neuron. 57, 634-660 (2008).
- Arenkiel, B. R., Ehlers, M. D. Molecular genetic and imaging technologies for circuit based neuroanatomy. Nature. 461, 900-907 (2009).
- Zhang, F. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439-456 (2010).
- Adamantidis, A. R., Zhang, F., Aravanis, A. M., Deisseroth, K., de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 450, 420-424 (2007).
- Sparta, D. R. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nature Protocols. 7, 12-23 (2012).
- Stuber, G. D. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 475, 377-380 (2011).
- Liu, X. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 484, 381-385 (2012).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone