Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Stress-induced Antibiotic Susceptibility Testing on a Chip
W tym Artykule
Podsumowanie
We have developed a microfluidic platform for rapid antibiotic susceptibility testing. Fluid is passed at high speeds over bacteria immobilized on the bottom of a microfluidic channel. In the presence of stress and antibiotic, susceptible strains of bacteria die rapidly. However, resistant bacteria can survive these stressful conditions.
Streszczenie
We have developed a rapid microfluidic method for antibiotic susceptibility testing in a stress-based environment. Fluid is passed at high speeds over bacteria immobilized on the bottom of a microfluidic channel. In the presence of stress and antibiotic, susceptible strains of bacteria die rapidly. However, resistant bacteria survive these stressful conditions. The hypothesis behind this method is new: stress activation of biochemical pathways, which are targets of antibiotics, can accelerate antibiotic susceptibility testing. As compared to standard antibiotic susceptibility testing methods, the rate-limiting step - bacterial growth - is omitted during antibiotic application. The technical implementation of the method is in a combination of standard techniques and innovative approaches. The standard parts of the method include bacterial culture protocols, defining microfluidic channels in polydimethylsiloxane (PDMS), cell viability monitoring with fluorescence, and batch image processing for bacteria counting. Innovative parts of the method are in the use of culture media flow for mechanical stress application, use of enzymes to damage but not kill the bacteria, and use of microarray substrates for bacterial attachment. The developed platform can be used in antibiotic and nonantibiotic related drug development and testing. As compared to the standard bacterial suspension experiments, the effect of the drug can be turned on and off repeatedly over controlled time periods. Repetitive observation of the same bacterial population is possible over the course of the same experiment.
Wprowadzenie
The rise of bacterial resistance intensifies the need for fast phenotype-based antibiotic susceptibility tests in order to safeguard our drugs of last resort. Standard susceptibility tests are based on bacterial growth inhibition in the presence of antibiotics that take multiple (8-24) hours to complete. We have developed a novel antibiotic susceptibility test on a microfluidic platform that relies on the stress-activation of biosynthetic pathways to accelerate the action of antibiotics.
Antibiotic susceptibility tests at the microfluidic scale carry the advantage of effective sample usage, since they require small numbers of bacteria. Additionally, microfluidic devices can be multiplexed in order to test multiple samples under multiple conditions1,2. Recently, a number of microfluidic methods for antibiotic susceptibility testing have been reported3-9. In these methods, bacteria are grown inside nano- and picoliter droplets3,7, in the full volume of the microfluidic channel4-6,8, or as single bacteria electrically localized to the bottom surface of the channel9. Although these tests are carried out in microfluidic channels, they all monitor microbial growth in the presence and absence of antibiotics similar to traditional methods. Growth measurements are taken via optical density, pH sensitive dyes, or bright field/phase contrast or fluorescence images. Although some of these tests are faster than traditional methods, they each passively detect antibiotic resistance. In other words, these methods still require the user to wait for bacterial growth as the final read-out.
In contrast, we have developed a method that uses a combination of shear and enzymatic stress to activate antibiotic-sensitive biochemical pathways10. Challenging the stressed bacteria with those antibiotics creates a more rapid susceptibility test. Bacteria that are resistant to the antibiotic are able to withstand the stressful conditions. Susceptible bacteria, on the other hand, are rapidly killed by the combined stresses. The percentage of cell death after one hour, measured by microscopy using a fluorescent dead cell stain, defines the phenotype of the bacteria (resistant vs. susceptible).
For successful implementation of our method, bacteria must be immobilized on the bottom surface of the microfluidic channel. In this way, bacteria can be subjected to various stresses and simultaneously imaged under a microscope in a single plane. A coated microscope glass slide is used for bacteria immobilization. The slide is precoated by the manufacturer with epoxide groups for nonspecific protein binding. The nonspecific binding of these epoxides to bacterial surface proteins covalently attaches the bacteria to the slide surface.
Strains are tested under identical conditions (shear + enzymatic stress) in the absence (control) and presence (experiment) of antibiotic. Phase contrast and fluorescence microscope pictures of each channel are taken automatically every two minutes for one hour. Resistance designations are then made by comparing the percent of dead bacteria in the experimental channel to those present in the control channel. After one hour, a sample with a cell death percentage greater than 1% is deemed susceptible, while less than 0.5% death is indicative of resistance. Percentages that fall between these two cut-offs are considered indeterminate and the sample must be tested again.
Microfluidic channels are defined in PDMS, which is a material of choice for microfluidic devices11. PDMS is optically transparent in a wide range of wavelengths, biocompatible, inert, permeable to gases and has low permeability to liquids; therefore it is well suited for these experiments.
Mechanical/shear stress is created by the flow of room temperature media over the immobilized bacteria. (Note: Warming the media to 37 °C has no significant effect on assay outcome.) Automated syringe pumps force media (containing dead cell stain +/- antibiotic, as well as optional enzymatic stressors) through the microfluidic channels (200 µm x 400 µm) at a flow rate of 1 ml/min to give 6.25 kPa of shear force or a shear rate of 6,000 sec-1. This rate equals or exceeds previously studied shear stresses on Staphylococci.
The enzyme, lysostaphin, was selected for preliminary experiments because it causes direct damage to the Staphylococcus cell wall. The concentration of lysostaphin (0.7 ng/ml) was sufficient to cause bacterial cell wall damage, but not sufficient to cause bacterial cell death without antibiotic in the time frame of the experiment. Lysostaphin is not required for the correct designation of bacterial susceptibility but it does augment the outcome, leading to increased cell death in susceptible strains. In contrast, shear stress is critical for assay function. When methicillin-sensitive Staphylococcus aureus strains are treated with lysostaphin and oxacillin in the absence of flow, no cell death is recorded over the course of the experiment.
Cell viability is monitored with a fluorescent dead cell stain12. The selection of the dye was based on its ability to selectively stain only damaged cells, its nontoxicity to live cells, and its low background fluorescence, which allowed for its direct addition to the cell media without additional steps. The selection of a fluorescent dye concentration of 0.25 µM was to achieve acceptable signal levels during a 1.6 sec exposure time to fluorescence excitation light.
The beta-lactam, oxacillin, was used in our preliminary studies. Methicillin-resistant S. aureus (MRSA) species are resistant to oxacillin and will not show any appreciable cell death in the time frame of the experiment. The concentration of 50 µg/ml was determined in the preliminary studies. Lower concentrations of antibiotic gave less separation between resistant and susceptible strains, while higher concentrations did not cause an appreciable difference in experimental outcomes.
We have previously reported on the successful development of a test that combines mechanical and enzymatic stresses that directly affect the bacterial cell wall13 with an antibiotic that inhibits cell wall biosynthesis14,15. These proof-of-principle experiments were carried out on a panel of MRSA and methicillin-sensitive S. aureus (MSSA). However, with the selection of proper experimental parameters, our method should be applicable to multiple species of bacteria and multiple classes of antibiotics.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Make the PDMS Layer (Figure 1)
- Vigorously mix PDMS and curing agent in a 10:1 ratio. To remove bubbles, degas the viscous mixture in a vacuum chamber for 1 hr at room temperature.
- On a scale, pour the PDMS slowly over the aluminum mold. Pour from the center and keep the mold leveled. Make sure to leave the pins uncovered. Stop pouring once target weight is achieved.
Our mold requires 4 g of PDMS and 0.4 g of curing reagent. - Level the mold inside an oven, and cure at 37 °C overnight.
Alternative curing times are 2 hr at 60 °C or 1 hr at 90 °C. - Dissect the cured PDMS layer along the edge of the mold and carefully peel it off of the mold surface with a pair of forceps. Clean the mold surface with 70% ethanol and a Q-tip.
2. Assemble the Flow Cell According to Figure 2
Standard assembly of PDMS with glass slides is done through oxygen plasma treatment of both surfaces, which ensures leak-free bonding between the PDMS and microscope glass slide. In the presented protocol, the plasma treatment would destroy the chemical coating on the glass slide. Therefore the slide is pressure-sealed rather than plasma treated.
- Place the glass window into the flow cell pocket.
- Lay a coated glass slide over the glass window inside the pocket of the flow cell with the active side up, and place the PDMS layer with channels facing down on top of it. Place the PDMS slide in such a way that channel inputs align with the through-holes in the metal plate. Gently push the air out from between the layers.
- Flip the PDMS/glass slide assembly so that the PDMS faces the glass window. Overlap the PDMS channel inputs with the through-holes in the metal plate.
- Place the pressure plate on top and tighten the screws.
- Place the assembled flow cell under the microscope. Set the microscope magnification to 60X and prealign the channel positions.
3. Prepare Log Phase Bacteria
- Day before the experiment: Inoculate 50 ml of Mueller Hinton broth containing 2% NaCl (MH2) with a bacterial colony. Shake at 250 rpm overnight at 37 °C.
One or two bacterial strains can be studied in one experiment for the described set-up. - Prior to experiment: Mix 50 µl of overnight bacteria culture into 50 ml of MH2 media. Shake at 250 rpm for 3 hr at 37 °C to ensure the bacteria are in log phase.
4. Warm the Experimental Solution Components at Least 10 Minutes Before They are Needed
- Thaw the fluorescent dye (5 mM stock) and lysostaphin (10 µg/ml stock) at room temperature.
- Warm the oxacillin powder to room temperature.
5. Prepare and Load the Bacterial Suspension
- After the end of 3 hr subculture: Take 10 ml of bacteria culture and centrifuge at 1,650 x g for 2 min.
- Remove the supernatant and resuspend bacteria in 1 ml of fresh MH2 media.
- Attach a short length of tubing to a 1 ml Luer lock syringe. Flush the syringe tubing with 1 ml of media. Leave a little bit of media in the tubing to avoid air bubbles when drawing in the bacterial suspension.
- Load 0.7 ml of bacteria type 1 into the syringe. Fill two channels of the flow cell with bacteria type 1. Watch for the liquid to appear on the other side of the channel after ca. 150 µl.
The transparency of the channel changes as it is filled with bacteria. - If experimenting with multiple bacteria types, repeat the loading procedure for bacteria type 2 into the two remaining channels of the flow cell.
- Place the flow cell inside the incubator at 37 °C for 45 min to allow bacterial settling and attachment to the slide surface.
6. Prepare and Load the Experimental Solutions
- Prepare 140 µl of 0.5 mM fluorescent dye solution by mixing 14 µl of fluorescent dye stock (5 mM) and 126 µl of MH2 media.
- Dilute 10 mg of oxacillin in 40 ml of MH2 media to obtain a final concentration of 250 µg/ml oxacillin.
- Prepare 130 ml of control solution with final concentrations of 0.25 µM fluorescent dye and 0.7 ng/ml of lysostaphin. To do so, mix 65 µl of fluorescent dye (0.5 mM), 9.12 µl of lysostaphin stock (10 µg/ml) and 130 ml of MH2 media.
- Prepare 130 ml of antibiotic solution with final concentrations of 0.25 µM fluorescent dye, 0.7 ng/ml of lysostaphin, and 50 µg/ml oxacillin. To do so, mix 65 µl of fluorescent dye (0.5 mM), 9.12 µl of lysostaphin stock (10 µg/ml), 26 ml of oxacillin (250 µg/ml) and 104 ml of MH2 media.
- Fill two 60 ml syringes with control solution and two 60 ml syringes with antibiotic solution. Keep solutions wrapped in aluminum foil to avoid light-induced degradation of the reagents.
Overfill the syringes to account for loss due to flushing of the tubing. - Remove air bubbles from the syringes by flicking. Attach and fill input tubing to the tip with the experimental solution.
- Mount syringes onto the pump. Place the syringe with the smallest volume first, then lock the plunger position. Fit the rest of the syringes onto the pump, squeezing their plungers as necessary.
- Set the pump speed to 1 ml/min and the pump volume to 60 ml. Do a flush with the pump until a steady stream of liquid is seen from all syringes.
7. Set Up the Flow Cell Under the Microscope
- Remove the flow cell from the centrifuge and mount it onto the microscope stage (Figure 3).
- Connect input/output tubing to each of the flow cell channels (one input/one output per channel).
Collecting output into four different containers allows for the measurement of individual channel output volumes.
8. Run the 60 Minute Experiment
- Check the prealigned positions from step 2.5. If the microscope field-of-view is not centered on the channel and/or is out-of-focus, adjust the settings and save the new positions.
Precise focusing may not be possible before flow start due to the high density of loaded bacteria. - Set the phase contrast acquisition time to 10 msec and the fluorescence acquisition time to 1,600 msec.
- Obtain phase contrast and fluorescence images for each position before initiating the flow.
This gives a qualitative estimate of the loaded bacterial density. - Start the liquid flow and immediately check that the microscope is focused on the bottom of the channels.
- Take phase contrast and fluorescence images of the target areas within the first minute of flow.
- Acquire images every 2 min after the first set of images until 60 min of flow has occurred. Refocus as necessary.
9. Disinfect the Flow Cell
- Make a 10% bleach solution in a beaker (100 ml). Fill 4 x 20 ml syringes with 10 ml of the mixture. Debubble the syringes and attach them to the flow cell.
It will take ~1-2 min for the channels to be clear of bacteria. - Set the pump speed to 1 ml/min and the pump volume to 3 ml. Run for 3 min.
- Fill 4 x 60 ml syringes with 60 ml of DI water. Debubble the syringes and attach them to the flow cell.
- Set the pump speed to 1 ml/min and the pump volume to 30 ml. Run for 30 min.
- Monitor the channel cleaning under the microscope.
- Disassemble the flow cell. Discard the used epoxy slide. Soak the flow cell components in DI water for 20 min. Air dry.
10. Analyze Images and Generate Data
- Count the number of bacteria in each image.
The open access software, CellProfiler is used to perform batch image processing16. A high level outline of the CellProfiler routine is summarized in Table 1. The number of bacteria present in the phase contrast image (Np) gives the total bacterial count. The number of bacteria visible in the fluorescence image (Nf) gives the number of dead bacteria. - Calculate the normalized bacterial cell death percentage as a function of time.
- Import Nf and Np for individual images into the data analysis software.
- Calculate the fraction of dead bacteria in each channel at a specific time point given by the fraction (Nf / Np) at t = T.
- Subtract the fraction of dead bacteria present at the outset of the experiment (t = 1 min) for both the control and the experimental channels.
- Subtract the fraction of dead bacteria present in the control channel from that present in the experimental channel at each time point with the following equation:
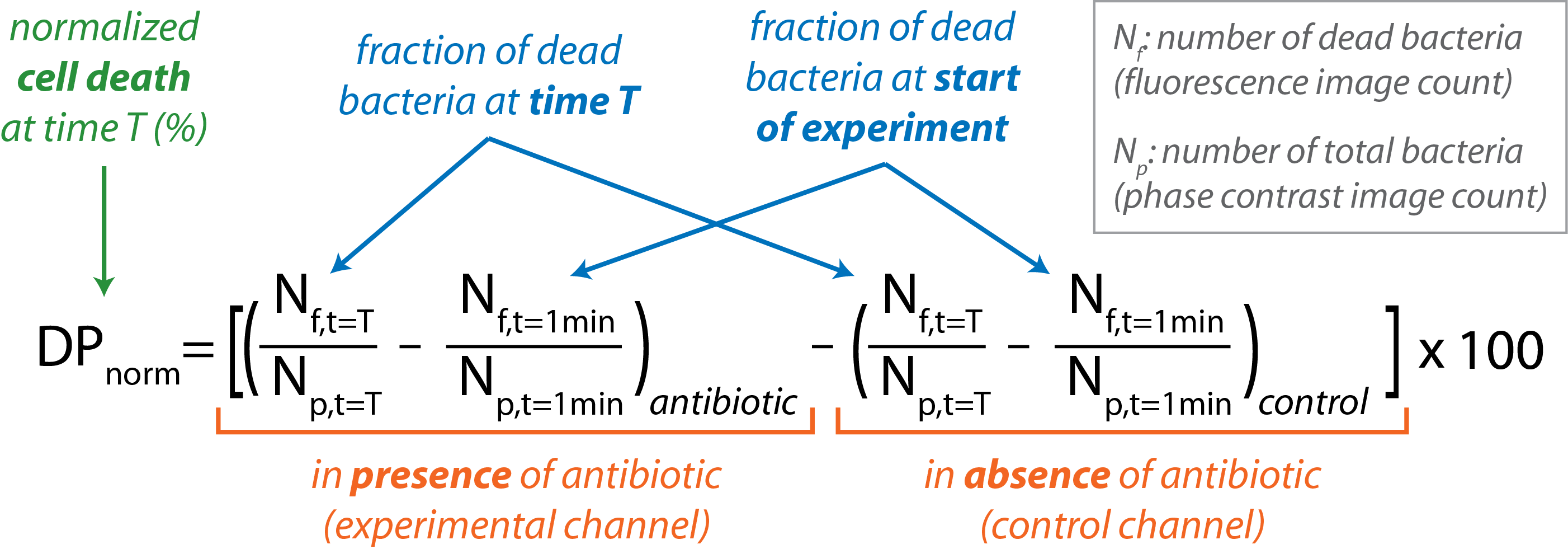
- Graph DPnorm vs. t for the course of the experiment.
Note that a sample with a cell death percentage greater than 1% is deemed susceptible, while less than 0.5% death is indicative of resistance. Percentages that fall between these two cut-offs are considered indeterminate and the sample must be tested again. - Use a spreadsheet to summarize and analyze results from different experiments.
Access restricted. Please log in or start a trial to view this content.
Wyniki
The data presented in Figure 4 show the response of a susceptible Staphylococcus aureus strain over time in an antibiotic-containing microfluidic channel. Phase contrast images acquired at 1 min and at the end of the 1 hr experiment are shown in Figures 4A and B. The analyzed 1 hr data are shown in Figure 4C with the bacteria highlighted in red (5,828 total). Corresponding fluorescence images are shown in Figures 4D
Access restricted. Please log in or start a trial to view this content.
Dyskusje
The presented protocol was validated and optimized in a set of experiments with methicillin-sensitive and methicillin-resistant Staphylococcus aureus strains10. Therefore, this protocol without modification should be directly applicable to other strains of S. aureus and other antibiotics with mechanisms of action affecting bacterial cell wall biosynthesis. Bacteria types other than S. aureus may require variation in the stress parameters: soluble (enzymatic) and mechanical stres...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The microfluidic method is patent pending: Sauer-Budge A, Sharon A, Kalashnikov M, Wirz H, inventors; Method and Device for Rapid Detection of Bacterial Antibiotic Resistance/Susceptibility patent PCT/US10/33523.
Podziękowania
We thank the engineers and students at the Fraunhofer Center for Manufacturing Innovation. For helping in the design, machining, and automation of the experimental system, we thank Andreas Prinzen, Holger Wirz, Doug Foss, David Chargin, and Dr. Sudong Shu. We thank Julia Kuckartz, Melanie Zimmermann, Niko Kraetzmar, Tim Gumbel, Josh Villanueva, Minori Shimizu, and Katarzyna Kuliga for help with testing experimental protocols and data collection. We acknowledge Drs. Anne E. Carpenter and Mark-Anthony Bray of the Imaging Platform at the Broad Institute of Harvard and MIT for help with development of the image analysis routine in CellProfiler. The project described was supported in part by Awards R21AI079474 and 1R01AI101446 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. The project was also supported by Fraunhofer USA.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| SYTOX Green | Invitrogen Corporation | S7020 | Dead cell fluorescence stain |
| Bovine Serum Albumin (BSA) | Sigma Aldrich, Inc | A9418-5G | Used for lysostaphin storage |
| Sodium Acetate | Sigma Aldrich, Inc | S8750-500G | Used for lysostaphin storage |
| Lysostaphin | Cell Sciences | CRL309A | Arrives as 1 mg solid. For storage: Dissolve in 20 mM sodium acetate. Mix with BSA solution to final concentration of 1% BSA and 100 µg/ml lysostaphin for storage |
| Oxacillin salt | Sigma Aldrich, Inc | 28221-1G | Antibiotic |
| Mueller Hinton Broth | Fisher Scientific | DF0757-17-6 | |
| Sodium chloride | Sigma Aldrixh | S3014-500G | 2% added to Mueller-Hilton broth prior to autoclaving |
| 1 ml, Luer-lock syringe | BD (Beckton, Dickinson and Comp.) | 14-823-2F | |
| 2 oz, Luer-lock syringe | BD (Beckton, Dickinson and Comp.) | 309653 - 60 mL | Overfill to ~65 ml |
| Microscope | |||
| Inverted Fluoresccence Microscope Olympus IX-70 | Cambridge Scientific | 9349 | |
| 60X, Fluorescence/Phase contrast objective | Olympus Corp. | LCPlan F1 60x/0.70 Ph2 | |
| Retiga 12-bit monochrome CCD camera | QImaging | RET-4000R-F-M-12-C | |
| Microscope automation | |||
| Shutters phase contrast/fluorescence | PRIOR Scientific | H204/H202 | |
| X/Y Stage | PRIOR Scientific | H107AENN | |
| Focus motor | PRIOR Scientific | H122 | |
| Joystick for XYZ control | PRIOR Scientific | CS152EF | |
| Proscan Controller | PRIOR Scientific | H3-XY2 | |
| Image Acquisition Software | Fraunhofer CMI | ||
| Flow Cell Assembly and PDMS | |||
| Flow Cell | BU Scientific Instruments Facility/Fraunhofer CMI | 3333-1044 | Engineering drawings were produced by Fraunhofer CMI |
| Glass window | Fraunhofer CMI | 3333-1054 | Glass window was cut to the proper size at Fraunhofer CMI |
| BOROFLOAT Window 50 mm x 50 mm | Edmund Optics Inc. | NT48-543 | |
| Sealing plate | BU Scientific Instruments Facility | 3333-1045 | |
| Epoxide glass slide | Arrayit Corporation | SuperEpoxy 2 | |
| PDMS master | Fraunhofer CMI | 3333-1053 | Master machined in aluminum or brass with UPM-0005 (ultrapresicion fly-cutting machine) |
| PDMS slide design | Fraunhofer CMI | 3333-1053 | |
| Tubing | |||
| Nut, Super flangeless Tinytight, headless, 1/16 in, PEEK, green | IDEX Health Science | M-644-03 | Flow cell inputs/outputs are tapped for this ferrule |
| Ferrule, Tinytight, 1/16 in, 6-40, .030 in TH, PEEK w/ SS lock ring, black | IDEX Health Science | M-657 | |
| Nut, Super flangeless Tinytight, headless, 1/16-1/32 in, 1/4-28, PEEK, natural | IDEX Health Science | P-255 | |
| Ferrule, Super Falngeless, 1/16 in, Tefzel (ETFE), yellow | IDEX Health Science | P-259 | Fits Luer-lock adapter |
| Tubing, Teflon FEP, .030 in x 1/16 in x 20 ft, green | IDEX Health Science | 1520G | |
| Adapter, quick connect female Luer to female 1/4-28, PEEK, red | IDEX Health Science | P-658 |
Odniesienia
- Mairhofer, J., Roppert, K., Ertl, P. Microfluidic systems for pathogen sensing: a review. Sensors. 9, 4804-4823 (2009).
- Yager, P., et al. Microfluidic diagnostic technologies for global public health. Nature. 442, 412-418 (2006).
- Boedicker, J. Q., Li, L., Kline, T. R., Ismagilov, R. F. Detecting bacteria and determining their susceptibility to antibiotics by stochastic confinement in nanoliter droplets using plug-based microfluidics. Lab Chip. 8, 1265-1272 (2008).
- Cao, J., et al. Uncovering toxicological complexity by multi-dimensional screenings in microsegmented flow: modulation of antibiotic interference by nanoparticles. Lab Chip. 12, 474-484 (2012).
- Chen, C. H., et al. Antimicrobial susceptibility testing using high surface-to-volume ratio microchannels. Anal. Chem. 82, 1012-1019 (2010).
- Churski, K., et al. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip. 12, 1629-1637 (2012).
- Eun, Y. J., Utada, A. S., Copeland, M. F., Takeuchi, S., Weibel, D. B. Encapsulating bacteria in agarose microparticles using microfluidics for high-throughput cell analysis and isolation. ACS Chem. Biol. 6, 260-266 (2011).
- Kim, K. P., et al. In situ monitoring of antibiotic susceptibility of bacterial biofilms in a microfluidic device. Lab Chip. 10, 3296-3299 (2010).
- Peitz, I., van Leeuwen, R. Single-cell bacteria growth monitoring by automated DEP-facilitated image analysis. Lab Chip. 10, 2944-2951 (2010).
- Kalashnikov, M., Lee, J. C., Campbell, J., Sharon, A., Sauer-Budge, A. F. A microfluidic platform for rapid, stress-induced antibiotic susceptibility testing of Staphylococcus aureus. Lab Chip. 12, 4523-4532 (2012).
- McDonald, J. C., Whitesides, G. M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 35, 491-499 (2002).
- Roth, B. L., Poot, M., Yue, S. T., Millard, P. J. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 63, 2421-2431 (1997).
- Francius, G., Domenech, O., Mingeot-Leclercq, M. P., Dufrene, Y. F. Direct observation of Staphylococcus aureus cell wall digestion by lysostaphin. J. Bacteriol. 190, 7904-7909 (2008).
- Jordan, S., Hutchings, M. I., Mascher, T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol. Rev. 32, 107-146 (2008).
- Koch, A. L. Bacterial wall as target for attack: past, present, and future research. Clin. Microbiol. Rev. 16, 673-687 (2003).
- Carpenter, A. E., et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
- Kohanski, M. A., Dwyer, D. J., Collins, J. J. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 8, 423-435 (2010).
- Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A., Collins, J. J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 130, 797-810 (2007).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone