Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Tissue-simulating Phantoms for Assessing Potential Near-infrared Fluorescence Imaging Applications in Breast Cancer Surgery
W tym Artykule
Podsumowanie
Near-infrared fluorescence (NIRF) imaging may improve therapeutic outcome of breast cancer surgery by enabling intraoperative tumor localization and evaluation of surgical margin status. Using tissue-simulating breast phantoms containing fluorescent tumor-simulating inclusions, potential clinical applications of NIRF imaging in breast cancer patients can be assessed for standardization and training purposes.
Streszczenie
Inaccuracies in intraoperative tumor localization and evaluation of surgical margin status result in suboptimal outcome of breast-conserving surgery (BCS). Optical imaging, in particular near-infrared fluorescence (NIRF) imaging, might reduce the frequency of positive surgical margins following BCS by providing the surgeon with a tool for pre- and intraoperative tumor localization in real-time. In the current study, the potential of NIRF-guided BCS is evaluated using tissue-simulating breast phantoms for reasons of standardization and training purposes.
Breast phantoms with optical characteristics comparable to those of normal breast tissue were used to simulate breast conserving surgery. Tumor-simulating inclusions containing the fluorescent dye indocyanine green (ICG) were incorporated in the phantoms at predefined locations and imaged for pre- and intraoperative tumor localization, real-time NIRF-guided tumor resection, NIRF-guided evaluation on the extent of surgery, and postoperative assessment of surgical margins. A customized NIRF camera was used as a clinical prototype for imaging purposes.
Breast phantoms containing tumor-simulating inclusions offer a simple, inexpensive, and versatile tool to simulate and evaluate intraoperative tumor imaging. The gelatinous phantoms have elastic properties similar to human tissue and can be cut using conventional surgical instruments. Moreover, the phantoms contain hemoglobin and intralipid for mimicking absorption and scattering of photons, respectively, creating uniform optical properties similar to human breast tissue. The main drawback of NIRF imaging is the limited penetration depth of photons when propagating through tissue, which hinders (noninvasive) imaging of deep-seated tumors with epi-illumination strategies.
Wprowadzenie
Breast-conserving surgery (BCS) followed by radiotherapy is the standard treatment for breast cancer patients with T1-T2 breast carcinoma1,2. Inaccuracies in intraoperative assessment of the extent of surgery result in positive surgical margins in 20 to 40% of the patients who underwent BCS, necessitating additional surgical intervention or radiotherapy3,4,5. Although extensive resection of adjacent healthy breast tissue might reduce the frequency of positive surgical margins, this will also hamper cosmetic outcome and increase comorbidity6,7. Novel techniques are therefore needed that provide intraoperative feedback on the location of the primary tumor and the extent of surgery. Optical imaging, in particular near-infrared fluorescence (NIRF) imaging, might reduce the frequency of positive surgical margins following BCS by providing the surgeon with a tool for pre- and intraoperative tumor localization in real-time. Recently, our group reported on the first in-human trial of tumor-targeted fluorescence imaging in ovarian cancer patients, showing the feasibility of this technique to detect primary tumors and intraperitoneal metastases with high sensitivity8. Before proceeding to clinical studies in breast cancer patients, however, the feasibility of various tumor-targeted NIRF imaging applications in BCS can already be evaluated preclinically using phantoms.
The following research protocol describes the use of NIRF imaging in tissue-simulating breast phantoms containing fluorescent tumor-simulating inclusions9. The phantoms provide an inexpensive and versatile tool to simulate pre- and intraoperative tumor localization, real-time NIRF-guided tumor resection, assessment of the surgical margin status, and detection of residual disease. The gelatinous phantoms have elastic properties similar to human tissue and can be cut using conventional surgical instruments. During the simulated surgical procedure, the surgeon is guided by tactile information (in the case of palpable inclusions) and visual inspection of the operative field. In addition, NIRF imaging is applied to provide the surgeon with real-time intraoperative feedback on the extent of surgery.
It should be emphasized that NIRF imaging requires the use of fluorescent dyes. Ideally, fluorescent dyes should be used that emit photons in the near-infrared spectral range (650 - 900 nm) to minimize absorption and scattering of photons by molecules physiologically abundant in tissue (e.g., hemoglobin, lipids, elastin, collagen, and water)10,11. Moreover, autofluorescence (i.e., the intrinsic fluorescence activity in tissues due to biochemical reactions in living cells) is minimized in the near-infrared spectral range, resulting in optimal tumor-to-background ratios11. By conjugating NIRF dyes to tumor-targeted moieties (e.g., monoclonal antibodies), targeted delivery of fluorescent dyes can be obtained for intraoperative imaging applications.
As the human eye is insensitive to light in the near-infrared spectral range, a highly sensitive camera device is required for NIRF imaging. Several NIRF imaging systems for intraoperative use have been developed so far12. In the current study, we used a custom build NIRF imaging system that was developed for intraoperative application in collaboration with the Technical University of Munich. The system allows for simultaneous acquisition of color images and fluorescence images. To improve the accuracy of the fluorescence images, a correction scheme is implemented for variations in light intensity in tissue. A detailed description is provided by Themelis et al.13
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Create Silicone Molds for Tumor-simulating Inclusions
- Collect solid items of the desired shape and size that can serve as models for tumor-simulating inclusions, e.g., beads or marbles.
- Thoroughly clean the tumor models. To ensure an easy removal from the silicone mold, the tumor models can be sprayed with anti-stick spray or covered with a thin layer of petroleum jelly or beeswax.
- Place each model in a separate thin walled square (plastic) box with a smooth surface. If necessary, fixate the model to the bottom of the box to keep it in position. Use a box that is slightly bigger than the tumor model itself to avoid wasting excessive amounts of silicone.
- Pour the required amount of silicone component A in a mixing bowl and add silicone component B in a 10:1 ratio by weight. Mix both components thoroughly. Optionally, a vacuum pump can be used to remove air bubbles from the silicone mixture.
- Gently pour the silicone mixture in the plastic box to prevent trapping air bubbles. The silicone mixture should be processed within 45 min to obtain optimal results.
- Let the silicone mixture solidify for at least 6 hr before cutting the mold and removing the tumor model. Optionally, the silicone mold can be cut in a zigzag pattern to allow it to fit back together cleanly. Maximum strength of the silicone is obtained after 3 days.
2. Create Tris-buffered Saline Solution
- Create a Tris-buffered saline (TBS) solution by adding 6.1 g (50 mM) Tris and 8.8 g (150 mM) NaCl to 800 ml deionized water.
- Add 1.0 g (15 mmol) of NaN3 to block oxygenation of hemoglobin (step 3.3 and 4.4) and to inhibit bacterial growth. CAUTION: NaN3 is a severe poison. It may be fatal in contact with skin or if swallowed. The toxicity of this compound is comparable to that of soluble alkali cyanides and the lethal dose for an adult human is about 0.7 g. Always follow the safety instructions as provided by the manufacturer.
- Adjust the pH to 7.4 and bring the volume to 1,000 ml with deionized water.
3. Create Fluorescent Inclusions
- Add 2 g agarose to 50 ml TBS from step 2. The higher melting point of agarose compared to gelatin (step 4.2) will prevent the inclusions from dissolving and leaking fluorescent dye when placed in melted gelatin. Optionally, the amount of added agarose can be altered to 1 or 3 g to obtain softer or palpable tumor inclusions, respectively.
- Heat the agarose slurry using a microwave until the boiling point is reached. Stir thoroughly until the agarose is completely dissolved.
- Add 1.1 g (17 µmol) hemoglobin and 5 ml intralipid 20% dissolved in 50 ml of TBS to the agarose mixture under constant stirring to resemble the optical characteristics of the surrounding breast phantom tissue (step 4).
- Add 20.0 mg (25.8 µmol) of the fluorescent dye indocyanine green to 83.8 ml deionized water. Make sure the dye is completely dissolved.
- Pipet 5.0 ml from this solution and add it to the agarose mixture to obtain a final concentration of 14 µM. Optionally, other fluorescent dyes than ICG can be used if desired with their own optimum concentration.
- Gently fill the silicone molds created in step 1 with the hot agarose mixture using a syringe (Figure 1A). Repeat this process until all molds are filled.
- Let the fluorescent inclusions solidify at RT for approximately one hr. Protect the inclusions from light by covering the entire mold with aluminum foil.
- After solidification, gently open the mold and press out the inclusion (Figure 1B). Optionally, use the tip of the syringe to apply small drops of melted agarose mixture on the surface of the inclusion. By repeating this process several times on the same location, small tumor spurs can be created to simulate infiltrative tumors.
- Protect the agarose inclusions from light and dehydration by wrapping them in aluminum foil and store them in a humidified storage container at 4 °C.
NOTE: The use of lower or higher fluorescent dye concentrations than the known concentration optimum will both result in diminished fluorescent signal intensity. The seemingly counterintuitive reduction in signal intensity with increasing dye concentrations above the optimum fluorescent dye concentration is due to a phenomenon known as quenching. When assessing the maximal depth penetration of a fluorescent dye in phantoms, using the optimal concentration is mandatory.
4. Create Breast Phantoms
- Obtain a cup-shaped mold to create breast phantoms of the desired size and volume, e.g., a glass or plastic bowl. The mold should have a smooth surface to prevent the gelatin form adhering to the mold. A mold volume of 500 ml will create breast phantoms of sufficient size.
- To create a breast phantom with a volume of 500 ml, add 50 g of gelatin 250 bloom to 500 ml TBS (step 2). Heat the gelatin slurry to 50 °C under constant stirring.
- Once the gelatin is completely dissolved, let the gelatin mixture gradually cool down and maintain it at a constant temperature of 35 °C using a hot water bath.
- Under constant stirring, add 5.5 g (85 mmol) bovine hemoglobin and 25 ml intralipid 20% to simulate absorption and scattering of photons in tissue, respectively.
- Prechill the cup-shaped mold at 4 °C for at least 1 hr. Next, pour the gelatin mixture in the mold to a level that corresponds to the predefined depth of the agarose tumor-simulating inclusion (Figure 1C). Let the gelatin mixture solidify at 4 °C for 30 min to one hr.
- After solidification, position a tumor-simulating fluorescent agarose inclusion on the surface of the phantom and temporarily fixate the inclusion with a small needle. Up to a maximum of three tumor-simulating fluorescent inclusions can be incorporated in a single breast phantom. Sufficient space (at least 5 cm) should be kept between individual tumor-simulating inclusions (Figure 1D).
- Pour the remainder of the warm gelatin mixture in the remaining mold volume, allowing for adherence of both layers without creating refraction artifacts. Mark the location of the fluorescent tumor-simulating inclusions on the mold. Let the phantom solidify O/N at 4 °C.
- Once solidified, remove the needles used for temporary fixation of the inclusions and gently remove the breast phantom from its mold (Figure 1E). Protect the breast phantom from light and dehydration by wrapping it in aluminum foil and store it in a humidified storage container at 4 °C.
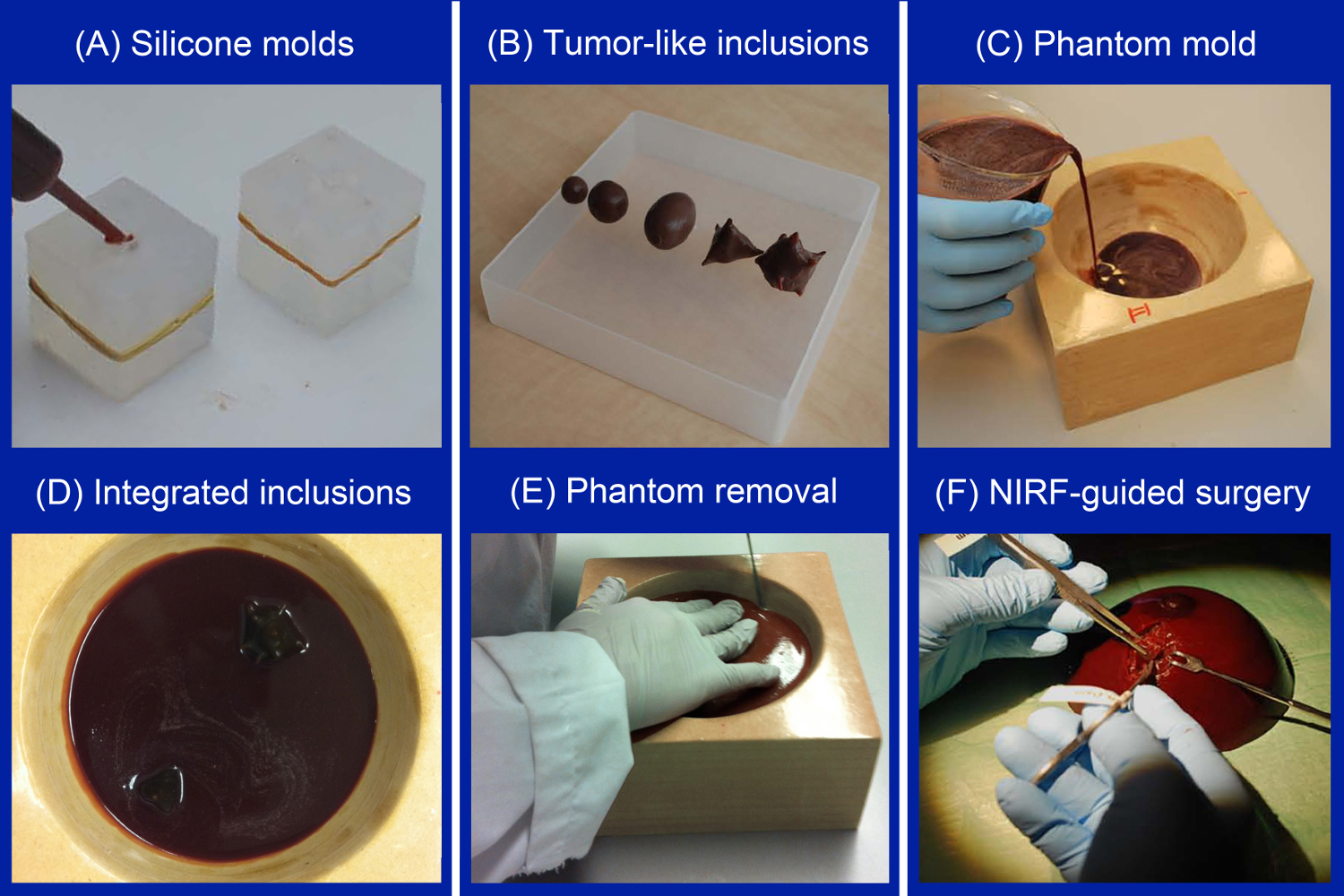
Figure 1. Sequential steps of creating breast phantoms containing fluorescent tumor-simulating inclusions. After creating silicone molds of the desired shape and size, the molds are filled with melted agarose mixture using a syringe (A). Tumor-simulating inclusions of differing size and shape were produced in the current study (B). Next, a thin layer of melted gelatin mixture is poured in a customized coated wooden breast mold (C). After solidification, the tumor-simulating inclusions are positioned, temporarily fixated, and covered with another layer of melted gelatin mixture (D). After solidification, the breast phantom is gently removed from its mold (E). The phantom can then be applied for simulating various NIRF imaging applications (F). Please click here to view a larger version of this figure.
5. Set the NIRF Camera System
- A NIRF camera system for intraoperative application is required for simulating targeted NIRF imaging in breast cancer surgery. Several NIRF imaging systems for real-time intraoperative NIRF imaging are currently available for investigational use. Although some differences between these devices exist, they all contain an excitation light source (for excitation of the fluorescent tumor inclusions) and a highly sensitive imaging device for detection of emitted photons.
- Make sure to use an excitation light source of a sufficient wavelength. For tumor-simulating inclusions containing ICG, use an excitation light source (e.g., laser) that emits photons between 750 and 800 nm. If an alternative fluorescent dye is used, the excitation wavelength should be adjusted conform the manufacturer’s instructions.
- In case the NIRF camera system contains an emission filter to filter out unwanted background signals, make sure that the correct filter is used. For tumor-simulating inclusions containing ICG, use an emission filter between 800 and 850 nm. Alternative fluorescent dyes may require different emission filters, depending on the manufacturers’ instructions.
NOTE: Make sure that there is zero overlap between the excitation and the emission wavelengths to prevent oversaturated images. In addition, the image acquisition time might have to be adjusted to obtain optimal fluorescent images. In the case of deep seated fluorescent inclusions or weak fluorescent signals, image acquisition time can be increased for up to several sec to min. In the case of superficial inclusions or strong fluorescent signals, acquisition time can be decreased to several msec to allow for video-rate fluorescence imaging in real-time.
6. Simulation of NIRF Imaging Applications in Breast Cancer Surgery
- Take the tissue-simulating breast phantom from its container and place it on a flat nonfluorescent surface. Next, position the NIRF imaging device above the breast phantom, leaving a sufficient working distance for excision of the tumor-simulating inclusions.
- Localize the tumor-simulating fluorescent inclusion using NIRF imaging and/or palpation of the phantom breast. In case no fluorescent signal can be detected, the inclusion is either positioned too deep in the phantom for detection or the image acquisition time should be increased.
- Once the inclusion is localized, incise the phantom breast and remove the tumor-simulating inclusion under real-time NIRF-guidance using conventional surgical instruments. Alternatively, the inclusion can be excised guided solely by visual inspection and palpation of the breast phantom to simulate the standard-of-care.
- Directly after removal of the tumor-simulating inclusion, image the surgical cavity for any remaining fluorescent activity indicating inadequate excision.
- In case of any remaining fluorescent activity, excise the inclusion remnant under direct NIRF guidance until no fluorescent signal is left.
- Image the excised phantom fragments to simulate NIRF-guided macroscopic margin status assessment. Hereto, slice the phantom tissue in 3 - 5 mm plaques and image the plaques accordingly. Fluorescence signal reaching into the surgical margins indicates the existence of positive surgical margins.
Access restricted. Please log in or start a trial to view this content.
Wyniki
Results from this study have been previously reported elsewhere9.
Our data show that NIRF imaging can be applied to detect fluorescent tumor-simulating inclusions in tissue-simulating breast phantoms, simulating NIRF-guided breast-conserving surgery in breast cancer patients. Using our phantom model, we found intraoperative tumor localization, NIRF-guided tumor resection, intraoperative assessment of surgical cavity margins, and detection of residual disease to be feasible (...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
We simulated potential clinical applications of NIRF-guided BCS through the use of breast-shaped phantoms with integrated tumor-simulating inclusions. Intraoperative tumor localization, NIRF-guided tumor resection, evaluation on the extent of surgery, and postoperative assessment of surgical margins were all found feasible using a custom-build NIRF camera system. Noninvasive detection of fluorescent tumor-simulating inclusions was only feasible for inclusions positioned in the phantom tissue at a depth of 2 cm or less. I...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by a grant from the Jan Kornelis de Cock foundation.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Bovine hemoglobin | Sigma-Aldrich, Zwijndrecht, The Netherlands | H2500 | Simulates absorption of photons in tissue |
| Intralipid 20% | Sigma-Aldrich, Zwijndrecht, The Netherlands | I141 | Simulates scattering of photons in tissue |
| Silicone A translucent 40 (2-components poly-addition silicone) | NedForm, Geleen, The Netherlands | Package consists of components A and B, that should be mixed one on one (A:B=10:1). Link to manufacturers page: http://tinyurl.com/ncjq7jx | |
| Gelatine 250 Bloom | Sigma-Aldrich, Zwijndrecht, The Netherlands | 48724 | Construction of breast-shaped phantoms |
| Agarose | Hispanagar, Burgos, Spain | Construction of tumor-simulating inclusions | |
| Tris | Sigma-Aldrich, Zwijndrecht, The Netherlands | T1503 | |
| HCl | Sigma-Aldrich, Zwijndrecht, The Netherlands | 258148 | |
| NaCl | Sigma-Aldrich, Zwijndrecht, The Netherlands | S9888 | |
| NaN3 | Merck, Darmstadt, Germany | 822335 | CAUTION: severe poison. The toxicity of this compound is comparable to that of soluble alkali cyanides and the lethal dose for an adult human is about 0.7 grams. |
| Examples of NIRF imaging devices for intraoperative application: | |||
| T2 NIRF imaging platform | SurgVision BV, Heerenveen, The Netherlands | Customized NIRF imaging system used in the current study. More details available at www.surgvision.com | |
| Photodynamic Eye | Hamamatsu Photonics Deutschland GmbH, Herrsching am Ammersee, Germany | PC6100 | www.iht-ltd.com |
| FLARE imaging system kit | The FLARE Foundation Inc, Wayland, MA, USA | www.theflarefoundation.org | |
| Fluobeam | Fluoptics, Grenoble, France | www.fluoptics.com | |
| Artemis handheld camera | Quest Medical Imaging BV, Middenmeer, the Netherlands | www.quest-mi.com | |
| Examples of NIRF fluorescent dyes for intraoperative application: | |||
| Indocyanine green | ICG-PULSION, Feldkirchen, Germany | PICG0025DE | Clinical grade fluorescent dye for NIRF imaging used in the current study. More details available at www.pulsion.com |
| IRDye 800CW NHS Ester | LI-COR Biosciences, Lincoln, NE, USA | 929-70021 | www.licor.com |
Odniesienia
- Bellon, J. R., et al. ACR Appropriateness Criteria® Conservative Surgery and Radiation - Stage I and II Breast Carcinoma. The Breast Journal. 17 (5), 448-455 (2011).
- Kaufmann, M., Morrow, M., Von Minckwitz, G., Harris, J. R. The Biedenkopf Expert Panel Members. Locoregional treatment of primary breast cancer. Cancer. 116, 1184-1191 (2010).
- Pleijhuis, R. G., et al. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. The Annals of Surgical Oncology. 16, 2717-2730 (2009).
- Singletary, S. E. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. American Journal of Surgery. 184 (5), 383-393 (2002).
- Jacobs, L. Positive margins: the challenge continues for breast surgeons. Annals of Surgical Oncology. 15 (5), 1271-1272 (2008).
- Krekel, N., et al. Excessive resections in breast-conserving surgery a retrospective multicentre study. The Breast Journal. 17 (6), 602-609 (2011).
- Wood, W. C. Close/positive margins after breast-conserving therapy: additional resection or no resection? Breast. 22, 115-117 (2013).
- Van Dam, G. M., et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nature Medicine. 17 (10), 1315-1319 (2011).
- Pleijhuis, R. G., et al. Near-infrared fluorescence (NIRF) imaging in breast-conserving surgery: assessing intraoperative techniques in tissue-simulating breast phantoms. European Journal of Surgical Oncology. 37 (1), 32-39 (2011).
- Baeten, J., Niedre, M., Dunham, J., Ntziachristos, V. Development of fluorescent materials for Diffuse Fluorescence Tomography standards and phantoms. Optics Express. 15 (14), 8681-8694 (2007).
- Luker, G. D., Luker, K. E. Optical imaging: current applications and future directions. Journal of Nuclear Medicine. 49 (1), 1-4 (2007).
- Keereweer, S., et al. Optical image-guided surgery - Where do we stand? Molecular Imaging Biology. 13 (2), 199-207 (2011).
- Themelis, G., Yoo, J. S., Soh, K. S., Shulz, R., Ntziachristos, V. Real-time intraoperative fluorescence imaging system using light-absorption correction. Journal of Biomedical Optics. 14 (6), 064012(2009).
- Themelis, G., et al. Enhancing surgical vision by using real-time imaging of αvβ3-integrin targeted near-infrared fluorescent agent. Annals of Surgical Oncology. 18 (12), 3506-3513 (2011).
- De Grand, A. M., et al. Tissue-like phantoms for near-infrared fluorescence imaging system assessment and the training of surgeons. Journal of Biomedical Optics. 11 (1), 014007(2006).
- Intes, X. Time-domain optical mammography SoftScan: initial results. Academic Radiology. 12 (10), 934-947 (2005).
- Kirsch, D. G., et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nature Medicine. 13 (8), 992-997 (2007).
- Tafreshi, N. K., et al. Noninvasive detection of breast cancer lymph node metastasis using carbonic anhydrases IX and XII targeted imaging probes. Clinical Cancer Research. 18 (1), 207-219 (2012).
- Nguyen, Q. T., Tsien, R. Y. Fluorescence-guided surgery with live molecular navigation - a new cutting edge. Nature Reviews Cancer. 13 (9), 653-662 (2013).
- Orosco, R. K., Tsien, R. Y., Nguyen, Q. T. Fluorescence imaging in surgery. IEEE Reviews in Biomedical Engineering. 6, 178-187 (2013).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone