Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Microfluidic Genipin Deposition Technique for Extended Culture of Micropatterned Vascular Muscular Thin Films
W tym Artykule
Podsumowanie
We present a method for microfluidic deposition of patterned genipin and fibronectin on PDMS substrates, allowing extended viability of vascular smooth muscle cell-dense tissues. This tissue fabrication method is combined with previous vascular muscular thin film technology to measure vascular contractility over disease-relevant time courses.
Streszczenie
The chronic nature of vascular disease progression requires the development of experimental techniques that simulate physiologic and pathologic vascular behaviors on disease-relevant time scales. Previously, microcontact printing has been used to fabricate two-dimensional functional arterial mimics through patterning of extracellular matrix protein as guidance cues for tissue organization. Vascular muscular thin films utilized these mimics to assess functional contractility. However, the microcontact printing fabrication technique used typically incorporates hydrophobic PDMS substrates. As the tissue turns over the underlying extracellular matrix, new proteins must undergo a conformational change or denaturing in order to expose hydrophobic amino acid residues to the hydrophobic PDMS surfaces for attachment, resulting in altered matrix protein bioactivity, delamination, and death of the tissues.
Here, we present a microfluidic deposition technique for patterning of the crosslinker compound genipin. Genipin serves as an intermediary between patterned tissues and PDMS substrates, allowing cells to deposit newly-synthesized extracellular matrix protein onto a more hydrophilic surface and remain attached to the PDMS substrates. We also show that extracellular matrix proteins can be patterned directly onto deposited genipin, allowing dictation of engineered tissue structure. Tissues fabricated with this technique show high fidelity in both structural alignment and contractile function of vascular smooth muscle tissue in a vascular muscular thin film model. This technique can be extended using other cell types and provides the framework for future study of chronic tissue- and organ-level functionality.
Wprowadzenie
Vascular diseases, such as cerebral vasospasm1,2, hypertension3, and atherosclerosis4, develop slowly, are typically chronic in nature, and involve dysfunctional force-generation by vascular smooth muscle cells (VSMCs). We aim to study these slow-progressing vascular dysfunctions using in vitro methods with finer control of experimental conditions than in in vivo models. We have previously developed vascular muscular thin films (vMTFs) for measuring functional contractility of in vitro engineered cardiovascular tissues5, but this method has been limited to relatively short-term studies. Here, we present a substrate modification technique that expands our previous vMTF technique for long-term measurements.
While the endothelium is also critical in overall vascular function, engineered arterial lamellae provide a useful model system for assessing changes in vascular contractility during disease progression. To engineer a functional vascular disease tissue model, both the structure and function of the arterial lamella, the basic contractile unit of the vessel, must be recapitulated with high fidelity. Arterial lamellae are concentric, circumferentially-aligned sheets of contractile VSMCs separated by sheets of elastin6. Microcontact printing of extracellular matrix (ECM) proteins onto polydimethylsiloxane (PDMS) substrates has been previously used to provide guidance cues for tissue organization to mimic aligned cardiovascular tissue5,7-10. However, tissues patterned using microcontact printing can lose integrity after 3-4 days in culture, limiting their applicability in chronic studies. This protocol provides a solution to this issue by replacing previous microcontact printing techniques with a new microfluidic deposition technique.
Genchi et al. modified PDMS substrates with genipin and found prolonged viability of myocytes up to one month in culture11. Here, we use a similar approach to extend culture of patterned vascular smooth muscle cells on PDMS. Genipin, a natural hydrolytic derivative of the gardenia fruit, is a desirable candidate for substrate modification due to its relatively low toxicity compared to similar crosslinking agents and its increasing use as a biomaterial in the fields of tissue repair12,13 and ECM modification14,15. In this protocol, fibronectin is utilized as a cell guidance cue, as in previous microcontact printing methods; however, genipin is deposited onto PDMS substrates prior to fibronectin patterning. Thus, as cells degrade the patterned matrix, newly synthesized ECM from attached VSMCs can bind to the genipin-coated PDMS substrate.
This protocol utilizes a microfluidic delivery device for two-step genipin and ECM deposition. The design of the microfluidic device mimics microcontact printing patterns used for engineered arterial lamellae in previous studies16. Thus, we expect this protocol to yield arterial lamellae mimics that successfully recapitulate the highly-aligned in vivo structure and contractile function of arterial lamellae. We also evaluate tissue contractility to confirm that genipin is a suitable substrate modification compound for long-term in vitro vascular disease models.
Access restricted. Please log in or start a trial to view this content.
Protokół
Note: The goal of this protocol is to construct and utilize a vascular muscular thin film (vMTF) with the structure shown in Figure 1 to assess contractility during extended culture of vascular smooth muscle cells (VSMCs) on PDMS substrates. To prolong VSMC viability, we utilize the crosslinker compound genipin. The substrates for these vMTFs are designed to analyze tissue contractility as developed by Grosberg et al.8 Other vMTF methods5 may also be used, with subtle changes to the presented substrate fabrication protocol.
1. Substrate Fabrication
- Coverslip Cleaning
- Place 25 mm diameter glass coverslips into a coverslip staining rack. Place the rack into a large beaker or container (e.g., an empty 100 - 1,000 µl pipet tip container).
- Add 70% ethanol to the container to fully immerse the coverslips. Sonicate them for at least 30 min.
- Remove the coverslip rack from the ethanol solution. Allow the coverslips to air dry by hanging the rack in a sterile culture hood (to prevent particle accumulation on coverslips) for 1- 2 hr.
Note: The coverslips must be completely dry prior to the following steps.
- Poly(N-iso-propylacrylamide) (PIPAAm) Strip Isolation on Coverslip
- Using adhesive tape, tape off the sides of a cleaned coverslip, leaving an exposed strip centered on the coverslip (Figure 1A). Modify the width of this exposed strip based on application and/or microfluidic design.
- Mark the edges of tape strips on the coverslip using a lab marker for later reference (Figure 1A).
- Cut around the perimeter of the coverslip to release from the dish (Figure 1A, dotted red line).
- Poly(N-iso-propylacrylamide) (PIPAAm) Coating
- Using an analytical balance, weigh 1 g of PIPAAm powder. Do not use unpolymerized N-iso-propylacrylamide, which is a carcinogen.
- Transfer the PIPAAm to a 50 ml centrifuge tube. Add 10 ml of 1-butanol inside a chemical hood to yield a 10% w/v solution. CAUTION: The flash point of 1-butanol is 37 oC. Store the resulting solution in a flammable cabinet and avoid heating.
- Allow the PIPAAm to dissolve for 10 min. If powder is still visible, mix the solution using a vortex-mixer until all powder dissolves.
Note: The following steps require the use of a spin coater. For each coverslip: - Place the taped coverslip on the spin coater chuck with forceps.
- Transfer 150 µl of PIPAAm solution onto the coverslip by placing droplets along the exposed glass in the center of the coverslip. Ensure complete coverage of the exposed area.
- Spin coat PIPAAm using the following recipe:
- Ramp 10 sec to 3,000 rpm. Dwell for 5 sec.
- Ramp 10 sec to 6,000 rpm. Dwell for 60 sec.
- Ramp 10 sec to 3,000 rpm. Dwell for 5 sec.
- Place the coverslip into a covered Petri dish with the PIPAAm facing up. Allow to air dry for at least 15 min.
- Carefully remove the adhesive tape from all coverslips, leaving the full coverslip exposed with a thin layer of PIPAAm coating in a center strip.
- PDMS Coating
- Mix and degas 15 g of PDMS in a 10:1 base:crosslinker ratio. Add 7 - 8 drops of sonicated 0.2 µm fluorescent microbeads prior to mixing. Cover the cup of PDMS with aluminum foil when not in use to prevent dust and other particles from contaminating the PDMS.
Note: The following steps require the use of a spin coater. For each coverslip: - Place a PIPAAm-coated coverslip on the spin coater chuck with forceps.
- Transfer PDMS onto the coverslip, covering at least one-third of the coverslip area.
- Spin coat using the following recipe:
- Ramp 5 sec to 500 rpm. Dwell 5 sec.
- Ramp 5 sec to 1,000 rpm. Dwell 5 sec.
- Ramp 10 sec to 3,000 rpm. Dwell 10 sec.
- Ramp 10 sec to 4,000 rpm. Dwell 60 sec.
- Ramp 10 sec to 2,000 rpm. Dwell 15 sec.
- Ramp 10 sec to 1,000 rpm. Dwell 10 sec.
- Ramp 5 sec to 500 rpm. Dwell 5 sec.
- Place the coverslip into a covered Petri dish with the PDMS facing up. Record the time when the coverslip was spin-coated. Keep track of the time associated with each coverslip throughout the experiment for later use in determining the PDMS substrate thickness.
- Place the Petri dish containing the coverslips in a 90 °C oven for at least 1.5 hr to ensure proper PDMS curing. If an oven is not available, let the coverslips cure for at least 48 hr at RT.
- Remove the coverslips from the oven and store them in a dark drawer until ready to use.
- Set aside every fourth coverslip for later measurement of substrate thickness, as a function of spin coating time, with a profilometer.
- Mix and degas 15 g of PDMS in a 10:1 base:crosslinker ratio. Add 7 - 8 drops of sonicated 0.2 µm fluorescent microbeads prior to mixing. Cover the cup of PDMS with aluminum foil when not in use to prevent dust and other particles from contaminating the PDMS.
2. Microfluidic Patterning for Engineering Tissues
- Fabrication of Microfluidic Devices
- Design of Tissue Microfluidic Photomask
- Use any appropriate computer-aided design program to design microfluidic patterns. For arterial lamellae composed of human umbilical cord artery vascular smooth muscle cells, use an alternating pattern of 10 µm channels with 10 µm walls.
- Use binary channel branching if possible17, but other branching designs may be used. Decrease the width and length of channels for each branching iteration until attaining the desired tissue pattern spacing (walls and channels, Figure 1B).
- Design the device to have a single inlet for surface treatment solution placement and a single outlet for application of vacuum.
- Fabricate a photomask containing the microfluidic design(s), as previously described18.
- Photolithographic Wafer Fabrication
Note: Perform photolithography in a suitable cleanroom or similar facility. To make silicon wafers with patterns for soft lithographic fabrication of tissue microfluidic devices (~20 - 25 µm channel height) using photolithography:- Clean a silicon wafer in acetone, methanol, and isopropyl alcohol for 1 min each. Dry the wafer with a nitrogen gun.
- Prebake the wafer on a hot plate for 5 min at 115 °C to remove excess moisture.
- Spin coat the wafer with SU-8 3025 photoresist using the following recipe to yield features 20 - 25 µm in height:
- Ramp 5 sec to 500 rpm. Dwell 5 sec.
- Ramp 15 sec to 4,000 rpm. Dwell 15 sec.
- Soft bake the wafer on a hot plate at 95 °C for 15 min.
- Load a photomask, and expose the wafer for 16 sec using a vacuum contact program on a contact mask aligner.
- Hard bake the wafer on a hot plate at 95 °C for 4 min.
- Develop the wafer for 6 min in developer. Then, wash the wafer twice for 2 sec in fresh developer and rinse the wafer with isopropyl alcohol.
- Silanate the patterned wafer O/N by placing 2 - 3 drops of tridecafluoro-trichlorosilane in an empty dish within a vacuum desiccator. Prop the wafer up using Petri dishes so that both the bottom and top of the wafer are exposed.
CAUTION: Tridecafluro-trichlorosilane is a flammable and corrosive liquid. Proper personal protective equipment and local exhaust is necessary for use.
- Tissue Microfluidic Device Fabrication
- Place a silanated, patterned wafer feature-side up in a Petri dish.
- Mix and degas 100 g of PDMS with a 10:1 base:crosslinker ratio. Pour the PDMS into the dish, completely and evenly covering the wafer.
- Place the dish in a vacuum desiccator until all air bubbles are removed from the uncured PDMS, approximately 30 min. Cure the PDMS in the dish at 90°C for at least 1.5 hr. Time and temperature can be adjusted as dictated by manufacture guidelines to obtain a complete cure.
- Once the PDMS has cured, cut out the PDMS around the wafer with a razor blade and carefully release the PDMS-covered wafer from the dish. Remove excess PDMS underneath wafer and slowly peel PDMS away from the top of wafer.
- Place the PDMS disk feature-side up in a clean dish and store the wafer away from light after use.
- Cut away the excess PDMS from around the patterns using a razor blade. Cut devices into rectangular shapes (Figure 1C) to ease peeling of device from substrates in later steps. Precise cuts are not necessary, as long as ample space exists for the inlet, outlet, and tissue pattern area (Figure 1C).
- Punch inlet and outlet holes (Figure 1C) using a 1 mm surgical biopsy punch.
- Design of Tissue Microfluidic Photomask
- Microfluidic Device Deposition
Note: In this protocol, microfluidic delivery is used to deposit patterned genipin, the key crosslinking agent for long-term tissue culturing, as well as fibronectin. Steps prior to penicillin/streptomycin sterilization (2.2.3) do not need to take place in sterile conditions, but limiting contamination and dust collection is encouraged throughout the protocol. All steps occurring after coverslip sterilization with penicillin/streptomycin (2.2.3) should utilize sterile technique. Note: This portion of the protocol must be started one day prior to cell seeding.- Substrate and Microfluidic Device Preparation
- Sonicate the microfluidic devices in 70% ethanol for at least 30 min.
- Dry the sonicated microfluidic devices using pressurized air or nitrogen, and place them in a Petri dish with channel features face-up to prevent unnecessary wear on the features.
- Place up to 10 vMTF substrate coverslips in a UVO cleaner (cover removed on dish so the surface is functionalized) for 8 min.
- Remove the UVO-treated coverslips, and place the microfluidic devices feature-side down onto each slip one at a time (orientation should be similar to Figure 1C). Press down firmly on the devices to ensure a tight seal to the PDMS-coated coverslips.
- Deposition of Genipin and Fibronectin
- Prepare a 5 mg/ml genipin solution by adding 1 ml of sterile ddH2O to a 5 mg container of lyophilized genipin. Mix the solution using a vortex-mixer. Set aside at RT for at least 30 min.
Note: The powder is difficult to solubilize at RT, so repetitive mixing for at least a minute is often necessary. - Quickly, place a drop of 70% ethanol at the inlet of each device for device priming. Note: The ethanol should wick through the devices.
- After 5 - 10 min, carefully aspirate the excess ethanol at the inlet, immediately replacing it with 1X phosphate buffered saline (PBS) at the inlet. From this point forward, be sure to not allow inlet to become completely dry to avoid introduction of air to the device.
- Place a vacuum aspirator tip at the outlet of each device. Draw 1X PBS through devices to rinse ethanol away. Leave a small amount of 1X PBS at the inlet. Note: If inlet appears nearly dry, add more 1X PBS.
- Aspirate excess 1X PBS so that only a small amount remains at the inlet prior to application of genipin solution.
- Place 60 μl of the 5 mg/ml genipin solution at each inlet (Figure 1D). Draw the genipin solution through the devices by placing a vacuum aspirator tip at the outlet (Figure 1D). Be sure not to draw all of the solution through, leaving a small amount of solution at the inlet.
- Place drops (approximately dime-sized) of 1X PBS at both the inlet and outlet to maintain wetting during incubation. Move the dish containing devices to a humidified oven or incubator (sterile environment is not necessary) set to 37 °C, and incubate for 4 hr. The dish does not need to be covered.
- During incubation, resuspend fibronectin to a concentration of 50 μg/ml in sterile ddH2O on ice for at least 30 min prior to application to microfluidic device.
- After the incubation of genipin, aspirate all remaining 1X PBS at the device outlets. Continue to apply a vacuum aspirator at each device outlet, pulling through the remaining 1X PBS at the inlet.
- Place 100 μl of 50 μg/ml fibronectin solution at each inlet, adding to a minimal amount of remaining 1X PBS at inlet (Figure 1D).
- Draw the fibronectin solution through the devices using a vacuum aspirator tip at the outlet (Figure 1D). Be sure to not draw all of the solution through. Note: Fibronectin drop will look different than the 1X PBS and genipin due to differences in surface tension.
- Move the uncovered dish containing the devices to an oven or incubator set to 37 °C, and incubate for 24 hr. Note: The fibronectin step does not require wetting of the inlet and outlet with 1X PBS. The remaining pool of fibronectin at the inlet will dry out. This is expected.
- Prepare a 5 mg/ml genipin solution by adding 1 ml of sterile ddH2O to a 5 mg container of lyophilized genipin. Mix the solution using a vortex-mixer. Set aside at RT for at least 30 min.
- Sterilization and Preparation for Cell Seeding
- Prepare a solution of penicillin/streptomycin for sterilization of patterned vMTF coverslips. Add 5 ml of penicillin/streptomycin (10,000 units/ml; 10,000 μg/ml) to 500 ml of sterile 1X PBS.
- Place the dish containing the devices in a sterile biosafety hood.
- Carefully remove the devices from the coverslips by slowly peeling the device at a corner, while lightly grasping the coverslip in opposite hand. Note: This step requires practice to reduce coverslip damage in the removal process. An alternative is using a syringe to inject 1X PBS at the inlet and/or outlet to aid in device release.
- Place the coverslips in sterile six-well dishes. Add at least 5 ml of penicillin/streptomycin solution to each well. Place the dishes in a sterile incubator at 37 °C for at least 30 min.
- After sterilization, aspirate the penicillin/streptomycin solution, and seed the coverslips with cultured human umbilical artery vascular smooth muscle cells19 (Figure 1D). The seeding concentration for VSMCs is ~80,000 cells per cm2. To reduce the number of cells needed for each sample, use a reducer to decrease the seeding area. One example of a reducer is the cut top of a 15 ml conical tube attached to the coverslip with sterile vacuum grease prior to seeding.
- Incubate the seeded coverslips in a sterile incubator at 37 °C and 5% CO2 and allow the seeded cells to attach and form aligned arterial lamellae mimics O/N (Figure 2A-B).
- Long-term vMTF Tissue Culture
- One day after seeding, remove the cell medium and reducers. Rinse the tissues with 1X PBS. Add 4 ml of serum-free cell medium to induce a contractile phenotype in the VSMCs20.
- Repeat the 1X PBS rinse and addition of fresh serum-free medium every other day as desired for long-term culture.
- Substrate and Microfluidic Device Preparation
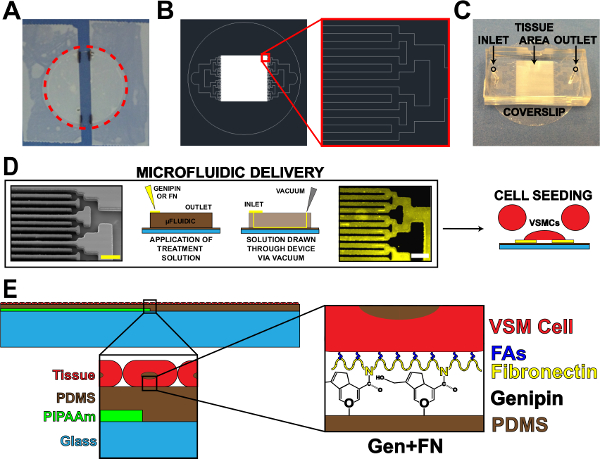
Figure 1. Microfluidic Protein Delivery Device. (A) Taped off coverslip for PIPAAm coating. Red dotted circle: cutting path to release coverslip. (B) Representative AutoCAD drawing of tissue microfluidic mask pattern. Inset: Detail of binary branching to alternating 10 µm x 10 µm tissue pattern. (C) Placement of microfluidic device on a coverslip substrate with inlet and outlet indicated. (D) Schematic of microfluidic protein patterning and delivery. Left-to-right: scanning electron microscope image of microfluidic channels (scale bar: 50 µm); Detailed schematic of method for protein deposition; Immunohistochemistry stained fibronectin (scale bar: 50 µm); Cell seeding with vascular smooth muscle cells. (E) Schematic of fabricated tissue. 1st inset: Detail of layered construct. 2nd inset: Detail of genipin modification of PDMS substrate after microfluidic deposition. © IOP Publishing. Reproduced and/or modified with permission. All rights reserved.19 Please click here to view a larger version of this figure.
3. Tissue Function Analysis with vMTF Contractility Assay
Note: The MTF contractility assay presented here is modeled after the technique developed in Grosberg et al.8
- vMTF Contractility Experiment
- Place a tissue sample in a 100 mm dish. Add sterile 1X Tyrode’s solution8 at pH 7.4 warmed to 37 °C to cover the sample.
- Use a razor blade to make several parallel cuts perpendicular to the PIPAAm edge. Make cuts in a manner that yields wider tissue sections that will be the vMTFs (with width ~2 mm) alternating with thin strips (Figure 3A, side cuts). To make clean cuts, place a razor blade in contact with the sample and firmly drag to the side.
- Rotate the dish 90° and make two straight, parallel cuts in the middle of the tissue, parallel to the strip of PIPAAm (Figure 3A, end cuts). Remove and dispose of the loose strip of tissue between these cuts and the thin strips in between vMTFs (cut in the previous step) to prevent adjacent films from making contact.
- Allow the sample to rest at RT for 10 min, or until all the PIPAAm has dissolved. Note: If PIPAAm remains in future steps, sample may be returned to the cutting dish to dissolve residual PIPAAm. A gentle scraping of the underside of vMTF can aid in PIPAAm removal, as needed.
- Place a small dot of vacuum grease in a clean 35 mm Petri dish. Add 5 ml of fresh, sterile 1X Tyrode’s solution at 37 °C. Transfer the coverslip with cut films from the 100 mm dish to the 35 mm dish, and press onto vacuum grease to prevent movement of the coverslip.
- Place the dish in a temperature-controlled platform on the stereomicroscope stage.
- Capture time-lapse transmitted and fluorescent light images at desired intervals (e.g., 30 sec) throughout treatment assay.
- Serially treat vMTFs with 50 nM endothelin-1 for 20 min (induced contraction) and 100 µM HA-1077 for 30 min (tissue relaxation). Add concentrated solutions of each treatment to the experimental dish containing 5 ml of sterile 1X Tyrode’s solution at specified time points, yielding the desired treatment concentration in the 5 ml volume. Make treatment additions during the interval between time-lapse image acquisitions to avoid capturing pipette in images.
- vMTF Contractility Analysis
- Using coverslips set aside in 1.4.8, measure PDMS substrate thickness with a profilometer21. Create a thickness vs. spin time curve for each set of coverslips. Use this curve to estimate the vMTF thickness for each coverslip used in a contractility experiment.
- Measure the vMTF projection lengths for each time point during the experiment, and calculate the associated radii of curvature (Figure 3B) using previously reported methods8.
- Calculate vMTF stress at each time point using previous vMTF methods5.
Note: Use the estimated vMTF thickness calculated from 3.2.1. Measure VSMC thickness using confocal images, as previously reported9. Obtain PDMS Young’s modulus from company data sheets.
Access restricted. Please log in or start a trial to view this content.
Wyniki
The primary goal of this work was to extend the viability of micropatterned VSMCs on hydrophobic PDMS substrates. This was accomplished by incorporating a microfluidic delivery system to deposit patterned genipin and fibronectin on PDMS (Figure 1). Deposition of ECM proteins using microfluidic delivery yielded high fidelity transfer of the channel pattern with bare PDMS between lines of genipin and fibronectin (Figure 1D). The attached cells (Figure 1E) form confluent mo...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Here, we present a protocol that builds upon previously developed vMTF technology, allowing extended experiment times more typical of chronic vascular disease pathways1,23,24. To accomplish this, we micropattern genipin, which has previously been shown to provide long-term functionalization of PDMS substrates11, using a microfluidic deposition technique to yield engineered arterial lamellae with improved vascular tissue viability for use in MTF contractility experiments. McCain et al. devel...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We acknowledge financial support from the American Heart Association Scientist Development Grant, 13SDG14670062 (PWA) and the University of Minnesota Doctoral Dissertation Fellowship (ESH). We also acknowledge the microfabrication resources of the Minnesota Nano Center (MNC) and the image processing resources of the University Imaging Centers (UIC), both at the University of Minnesota. Parts of this work were carried out in the Characterization Facility, University of Minnesota, which receives partial support from NSF through the MRS program.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Coverslip staining rack | Electron Microscopy Sciences | www.emsdiasum.com/ | 72239-04 |
| Microscope cover glass - 25 mm | Fisher Scientific, Inc. | www.fishersci.com | 12-545-102 |
| Poly(N-iso-propylacrylamide) (PIPAAm) | Polysciences, Inc. | www.polysciences.com/ | #21458 |
| 1-butanol | Sigma-Aldrich | www.sigmaaldrich.com | 360465 |
| Spincoater | Specialty Coating Systems, Inc. | www.scscoatings.com | |
| Polydimethylsiloxane (PDMS) | Ellsworth Adhesives (Dow Corning) | www.ellsworth.com | 184 SIL ELAST KIT 0.5KG |
| Fluorescent microbeads | Polysciences, Inc. | www.polysciences.com/ | 17151 |
| Silicon wafers | Wafer World, Inc. | www.waferworld.com | 2398 |
| Photoresist | MicroChem Corp. | www.microchem.com | |
| Contact mask aligner | Suss MicroTec | www.suss.com | |
| Developer | MicroChem Corp. | www.microchem.com | |
| Tridecafluro-trichlorosilane | UCT Specialties, Inc. | www.unitedchem.com | T2492 |
| Surgical biopsy punch | Integra LifeSciences Corp. | www.miltex.com | 33-31AA-P/25 |
| Genipin | Cayman Chemical | www.caymanchem.com | 10010622 |
| 1X phosphate buffered saline | Mediatech, Inc. | www.cellgro.com | 21-031-CV |
| Fibronectin | Corning, Inc. | www.corning.com | 356008 |
| Penicillin/streptomycin | Life Technologies, Inc. | www.lifetechnologies.com | 15140-122 |
| Umbillical artery smooth muscle cells | Lonza | www.lonza.com | CC-2579 |
| Tyrode's solution components | Sigma-Aldrich | www.sigmaaldrich.com | various |
| Stereomicroscope | Zeiss | www.zeiss.com | 4350020000000000 |
| Temperature-controlled platform | Warner Instruments | www.warneronline.com | 641659; 640352; 641922 |
| Endothelin-1 | Sigma-Aldrich | www.sigmaaldrich.com | E7764-50UG |
| HA-1077 | Sigma-Aldrich | www.sigmaaldrich.com | H139-10MG |
Odniesienia
- Humphrey, J. D., Baek, S., Niklason, L. E. Biochemomechanics of cerebral vasospasm and its resolution: I. A new hypothesis and theoretical framework. Ann. Biomed. Eng. 35, 1485-1497 (2007).
- Hald, E. S., Alford, P. W. Smooth muscle phenotype switching in blast traumatic brain injury-induced cerebral vasospasm. Transl. Stroke Res. 5, 385-393 (2014).
- Olivetti, G., Anversa, P., Melissari, M., Loud, A. V. Morphometry of medial hypertrophy in the rat thoracic aorta. Lab. Invest. 42, 559-565 (1980).
- , Atherosclerosis. Nature. 407, 233-241 (2000).
- Alford, P. W., Feinberg, A. W., Sheehy, S. P., Parker, K. K. Biohybrid thin films for measuring contractility in engineered cardiovascular muscle. Biomaterials. 31, 3613-3621 (2010).
- Rhodin, J. A. G. Architecture of the vessel wall. Physiol. Rev. ed, B. erne,R. ., , American Physiology Society. (1979).
- Balachandran, K., et al. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc. Natl. Acad. Sci. U. S. A. 108, 19943-19948 (2011).
- Grosberg, A., Alford, P. W., McCain, M. L., Parker, K. K. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. 11, 4165-4173 (2011).
- Alford, P. W., Nesmith, A. P., Seywerd, J. N., Grosberg, A., Parker, K. K. Vascular smooth muscle contractility depends on cell shape. Integr. Biol. (Camb). 3, 1063-1070 (2011).
- Win, Z., et al. Smooth muscle architecture within cell-dense vascular tissues influences functional contractility). Integr. Biol. (Camb). , (2014).
- Genchi, G. G., et al. Bio/non-bio interfaces: a straightforward method for obtaining long term PDMS/muscle cell biohybrid constructs). Colloid Surface B. 105, 144-151 (2013).
- Fessel, G., Cadby, J., Wunderli, S., van Weeren, R., Snedeker, J. G. Dose- and time-dependent effects of genipin crosslinking on cell viability and tissue mechanics - Toward clinical application for tendon repair. Acta Biomater. , (2013).
- Lima, E. G., et al. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J. Biomed. Mater. Res. A. 91, 692-700 (2009).
- Madhavan, K., Belchenko, D., Tan, W. Roles of genipin crosslinking and biomolecule conditioning in collagen-based biopolymer: Potential for vascular media regeneration. J. Biomed. Mater. Res. A. , (2011).
- Satyam, A., Subramanian, G. S., Raghunath, M., Pandit, A., Zeugolis, D. I. In vitro evaluation of Ficoll-enriched and genipin-stabilised collagen scaffolds. J. Tissue Eng. Regen. Med. , (2012).
- Alford, P. W., et al. Blast-induced phenotypic switching in cerebral vasospasm. Proc. Natl. Acad. Sci. U. S. A. 108, 12705-12710 (2011).
- Song, H., Tice, J. D., Ismagilov, R. F. A microfluidic system for controlling reaction networks in time. Angew. Chem. Int. Ed. Engl. 42, 768-772 (2003).
- Whitesides, G. M., Ostuni, E., Takayama, S., Jiang, X., Ingber, D. E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 3, 335-373 (2001).
- Hald, E. S., Steucke, K. E., Reeves, J. A., Win, Z., Alford, P. W. Long-term vascular contractility assay using genipin-modified muscular thin films. Biofabrication. 6, 045005(2014).
- Han, M., Wen, J. K., Zheng, B., Cheng, Y., Zhang, C. Serum deprivation results in redifferentiation of human umbilical vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 291, C50-C58 (2006).
- Feinberg, A. W., et al. Muscular thin films for building actuators and powering devices. Science. 317, 1366-1370 (2007).
- Volfson, D., Cookson, S., Hasty, J., Tsimring, L. S. Biomechanical ordering of dense cell populations. Proc. Natl. Acad. Sci. U. S. A. 105, 15346-15351 (2008).
- Intengan, H. D., Schiffrin, E. L. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 38, 581-587 (2001).
- Kayembe, K. N., Sasahara, M., Hazama, F. Cerebral aneurysms and variations in the circle of Willis. Stroke. 15, 846-850 (1984).
- McCain, M. L., Agarwal, A., Nesmith, H. W., Nesmith, A. P., Parker, K. K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials. 35, 5462-5471 (2014).
- Weir, B., Grace, M., Hansen, J., Rothberg, C. Time course of vasospasm in man. 48, 173-178 (1978).
- McCain, M. L., Sheehy, S. P., Grosberg, A., Goss, J. A., Parker, K. K. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc. Natl. Acad. Sci. U. S. A. 110, 9770-9775 (2013).
- Agarwal, A., Goss, J. A., Cho, A., McCain, M. L., Parker, K. K. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab. Chip. 13, 3599-3608 (2013).
- Huh, D., Torisawa, Y. S., Hamilton, G. A., Kim, H. J., Ingber, D. E. Microengineered physiological biomimicry: organs-on-chips. Lab. Chip. 12, 2156-2164 (2012).
- Meer, A. D., van den Berg, A. Organs-on-chips: breaking the in vitro impasse. Integr. Biol. (Camb). 4, 461-470 (2012).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone