Method Article
Multi-exon Skipping Using Cocktail Antisense Oligonucleotides in the Canine X-linked Muscular Dystrophy
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Exon skipping is currently a most promising therapeutic option for Duchenne muscular dystrophy (DMD). To expand the applicability for DMD patients and to optimize the stability/function of the resulting truncated dystrophin proteins, a multi-exon skipping approach using cocktail antisense oligonucleotides was developed and we demonstrated systemic dystrophin rescue in a dog model.
Streszczenie
Duchenne muscular dystrophy (DMD) is one of the most common lethal genetic diseases worldwide, caused by mutations in the dystrophin (DMD) gene. Exon skipping employs short DNA/RNA-like molecules called antisense oligonucleotides (AONs) that restore the reading frame and produce shorter but functional proteins. However, exon skipping therapy faces two major hurdles: limited applicability (up to only 13% of patients can be treated with a single AON drug), and uncertain function of truncated proteins. These issues were addressed with a cocktail AON approach. While approximately 70% of DMD patients can be treated by single exon skipping (all exons combined), one could potentially treat more than 90% of DMD patients if multiple exon skipping using cocktail antisense drugs can be realized. The canine X-linked muscular dystrophy (CXMD) dog model, whose phenotype is more similar to human DMD patients, was used to test the systemic efficacy and safety of multi-exon skipping of exons 6 and 8. The CXMD dog model harbors a splice site mutation in intron 6, leading to a lack of exon 7 in dystrophin mRNA. To restore the reading frame in CXMD requires multi-exon skipping of exons 6 and 8; therefore, CXMD is a good middle-sized animal model for testing the efficacy and safety of multi-exon skipping. In the current study, a cocktail of antisense morpholinos targeting exon 6 and exon 8 was designed and it restored dystrophin expression in body-wide skeletal muscles. Methods for transfection/injection of cocktail oligos and evaluation of the efficacy and safety of multi-exon skipping in the CXMD dog model are presented.
Wprowadzenie
Duchenne muscular dystrophy (DMD) is an X-linked recessive muscle disease characterized by progressive muscle weakness, first described by Dr. Guillaume-Benjamin-Amand Duchenne (de Boulogne) 1. DMD is a common genetic disease affecting about 1 in 3,500 boys worldwide, with approximately 20,000 affected children born each year 2,3. Motor development is delayed and gait disturbances are seen in early childhood 4, followed by wheelchair dependency at about the early teens. Death typically occurs between the ages of 20 and 30 due to respiratory or cardiac failure 5-8. There is currently no cure for DMD. Treatment with glucocorticoids can slow the progression of muscle degeneration to some degree but is associated with significant side effects, including obesity and diabetes mellitus 2,7,8. DMD results from mutations in the dystrophin (DMD) gene, leading to a loss of functional dystrophin protein. DMD is an extremely large gene with over 2 million base pairs and 79 exons 9,10. Deletion, nonsense, and duplication mutations leading to out-of-frame mutations are the most common cause of the DMD phenotype. The regions of exons 3 - 9 and exons 45 - 55 are termed "mutation hotspots" as most patients have deletion mutations within these portions of the gene, leading to non-functional dystrophin in DMD patients 3,9,11-16. Dystrophin functions within the dystrophin-glycoprotein complex (DGC), which has a major role in muscle membrane stabilization. The N- and C-termini are the most important domains for function, while the central rod domain plays a less important role 3,9,17. The observance of a mild phenotype associated with Becker muscular dystrophy (BMD), which mostly results from in-frame mutations within the DMD gene, inspired the application of exon skipping for treating DMD. BMD patients have a shortened, but functional, dystrophin protein that maintains both termini 3,6,18. Exon skipping, in theory, can restore the reading frame, resulting in shortened-but-functional dystrophin proteins similar to those seen in BMD 3,19.
Multiple types of antisense oligonucleotides (AONs) have been tested in clinical trials, including 2'O-methylated phosphorothioates (2'OMePS) and phosphorodiamidate morpholino oligomers (PMOs). Skipping exons 51 and 53 using these AONs has been examined and while results are promising, single-exon skipping has limited applicability, as it is mutation-specific 3, 19, 20,21, 22-26. Questions also remain about the stability of the resulting shortened dystrophin proteins produced from single-exon skipping 22,23. Additionally, some patients require more than a single exon to be skipped in order to restore the reading frame 3. While technically more difficult, multi-exon skipping is one method that could address these problems 3,19. Multi-exon skipping has previously been demonstrated in dystrophic dog and human cell lines in vitro. Additionally, mdx52 mouse and canine X-linked muscular dystrophy (CXMD) dog models have been used for in vivo studies 22,24-27. Canine X-linked muscular dystrophy Japan (CXMDJ) beagles were used here, as the reading frame of CXMDJ can be restored by multi-exon skipping of exons 6 and 8, or additional exons (e.g., exons 3 - 9) (Figure 1). Beagle-based CXMD shares the same mutation pattern as the Golden Retriever muscular dystrophy (GRMD) model, but beagles are smaller and cheaper to maintain due to their body size, thus providing a useful model for DMD 28,29. CXMD dogs more closely mimic the human DMD phenotype than smaller animal models, like rodent, and are more reliable for toxicological assessments 3,22,30,31 (Figure 2). CXMD dogs display progressive muscle decay, gait disturbances, and cardiac and respiratory problems similar to those seen in DMD. Compared with single-exon skipping, multi-exon skipping is applicable to a much larger proportion of patients. Among the three most common mutation types (deletions, nonsense, and duplications), 80 - 98% of patients could be treated through multi-exon skipping 14,32,33, while 45% of all DMD patients could benefit from specifically skipping exons 45 - 55 3,19,22,34.
With the development of modified morpholinos, the efficiency of AON cocktails at facilitating exon skipping has improved. Arginine-rich cell-penetrating peptide-conjugated PMOs (PPMOs), and vivo-morpholinos (vPMOs) are AON chemistries that have significantly improved cell-penetrating ability and stability 3,35-38. Concerns remain about long-term AON toxicity; however, significant progress has been made. Chemical modifications made to morpholinos greatly decrease off-target effects and pre-clinical studies have reported no significant toxic effects 3,22,39,40. A remaining challenge for multi-exon skipping is the current requirement for each single AON to be tested for toxicity alone, as a single drug, instead of together as a cocktail 3,19,22,41,42. In DMD studies involving both single and multi-exon skipping targeted to the heart, there has been little improvement in dystrophic heart tissue. The efficacy of morpholinos in the heart is thought to be low because of poor cell-penetrating ability. Peptide-conjugated PPMOs have improved the ability of AONs to penetrate cardiac cells, increasing the amount of functional dystrophin protein rescued in the heart 3,19,38.
Here, our AON cocktail approach is discussed at length, including the design of AON sequences using ESEfinder software 43. Protocols for dog experiments with multi-exon skipping are also described. CXMDJ beagles were used for exons 6 and 8 skipping experiments. Multi-exon skipping in the CXMD dog model shows promising results, but challenges remain that need to be overcome before they are clinically applicable.
Protokół
All protocols listed below are in accordance with the animal care guidelines set forth by the National Center of Neurology and Psychiatry (NCNP) in Japan. All experiments were approved by the Institutional Animal Care and Use Committee of the NCNP.
1. Design of Antisense Oligos
- Use rescue ESE and ESEfinder programs to detect ESE sites 44, 45-47.
- Design 25 base pair (bp) sequences that are antisense to exons that are to be targeted. Target exons 6 and 8 in the dog model (Figure 1).
- Use 25 - 30 bp sequences for PMOs (Table 1) or 25 bp for 2'OMePS. To design 25 bp sequences, select sequences that are within the target region. Consider secondary structure, avoidance of heterodimers, exon splicing enhancer motifs, and GC content to create stable sequences 43. AONs should target at least one of the ESE sites as identified by the above-mentioned software.
- Select AONs with a GC content that is less than 65%, with fewer than 4 consecutive 'G's, and do not contain self-complementary sequences. Use NCBI Blast software to predict off-target annealing sites 22,48,49.
- Select an appropriate AON backbone chemistry. For in vitro experiments use 2'O-methyl oligonucleotides (2'OMePS) or morpholinos. For in vivo experiments, use 2'OMePS, morpholinos or vPMOs.3,19,48,50
2. In Vitro Experiments (Exons 6 and 8 Skipping in the CXMD Model)
- 2'OMePS Transfection of Dog Myoblasts
- Culture CXMD myoblasts in 3 ml of growth medium in 6-well plates. Seed 1 - 5 x 103 cells/cm2 with 0.5 ml/cm2 of medium for myoblasts. For the growth medium, use Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) 40.
- Incubate at 37 °C until 60 - 80% confluent. This takes approximately 12 to 24 hr.
- Dilute a cationic liposome transfecting agent to a total of 100 µl in reduced serum medium (2:1 ratio transfecting agent: AONs, e.g., 10 µl lipofectin vs. 5 µg AONs). Allow mixture to stand at RT for 30 - 45 min.
- Dilute AONs (2'OMePS or morpholinos) to a final volume of 100 µl in reduced serum medium.
- Combine the diluted transfecting agent with diluted AONs and incubate at RT for 10 - 15 min.
- While the transfection agent/AON mixture is sitting at RT, remove old media from cells via aspiration and wash cells with the media.
- Add 0.8 ml media to the transfection agent/AON mixture and then add the entire solution to the freshly-washed cells. Incubate for 3 hr at 37 °C.
- After incubating, replace media with differentiation media (DM); differentiation can take up to 10 days. Check to see whether differentiation has occurred starting at around day 3. The differentiation media is DMEM with 2% horse serum, 200 U/ml penicillin, 200 mg/ml streptomycin, and 10 µg/ml insulin.
- Morpholino Transfection of Dog Myoblasts
- Culture CXMD myoblasts in growth medium as described in step 2.1.
- Switch to differentiation medium (DM), and add 0.1 mM stock morpholino to each well to make the final concentration 1 µM. Heat cocktail morpholinos at 65 °C for 10 min before transfection or injection to avoid AON aggregation. Add a peptide delivery reagent 39,51 and adjust to a final concentration of 3 - 6 µM.
- After 16 - 48 hr of incubation, collect cells for RNA extraction. Add 1 ml acid guanidinium thiocyanate-phenol-chloroform to the cells to detach cells from the plate. Perform RNA extraction after this step.
- Alternatively, add trypsin so that it covers all cells and incubate for 2 min at 37 °C.
Note: If intending to perform immunochemistry, use chambered slide glasses for culturing. After differentiation, cells can be fixed using paraformaldehyde (PFA) (4% for 10 min).
- Alternatively, add trypsin so that it covers all cells and incubate for 2 min at 37 °C.
- RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
- Once cells are differentiated into myotubes, remove medium and add 1 ml acid guanidinium thiocyanate-phenol-chloroform; incubate for 10 min at RT.
- Transfer to 1.5 ml tubes. Combine with 200 µl chloroform and incubate at RT for 2 min until three separate layers can be seen. The three layers from top to bottom are: an RNA layer, a DNA layer, and a protein layer.
- Centrifuge at 12,000 × g for 15 min at 4 °C. Remove the top layer supernatant and place in a tube with 500 µl isopropanol (if stopping here, store the supernatant at -80 °C). Centrifuge the supernatant at 12,000 × g for 10 min at 4 °C; after centrifuging, keep the resulting RNA pellet and discard the supernatant.
- Wash the pellet with ethanol and centrifuge at 8,000 x g for 5 min at 4 °C. Evaporate residual ethanol by inverting the tube for 15 min and then add 15 - 30 µl of RNase-free water. Quantify total RNA concentration using US/VIS spectroscopy at 260 nm.
- Combine the required reagents for an RT-PCR reaction: 1.5 µl 10 μM forward primer, 1.5 µl 10 μM reverse primer, 1 µl dNTP, 5 µl one-step PCR kit buffer, 0.7 µl RNase inhibitor, 1 µl enzyme mixture from one-step PCR kit, and 200 ng of RNA. Once these have been mixed, add water to a final volume of 25 µl.
- Place mixture in a thermo-cycler. Run a 30 min cycle at 50 °C, a 15 min cycle at 95 °C, then 35 cycles of 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 1 min. Lastly, run a 10 min cycle at 72 °C. Store PCR product in a refrigerator at 4 °C or -20 °C
- Complementary DNA (cDNA) Sequencing
- Identify exon 6 - 9-skipped bands using agarose gel electrophoresis. Load 5 µl of each sample into the wells of a 1.5% agarose gel, run 135 V through the gel for 5 min, and then 120 V for 20 min. Next, incubate the gel in DNA gel stain at RT for 30 min. Visualize the bands using imaging software.
- Excise the band of interest using a gel extraction kit.
- Excise the DNA fragment and solubilize the gel slice using 200 µl NTI/100 mg gel. Let this sit for 5 to 10 min in a 50 °C water bath. Then transfer to silica membrane tube.
- Centrifuge at 11,000 x g for 30 sec. Wash twice with 700 µl NT3 buffer before centrifuging again at 11,000 x g for 30 sec.
- Dry the silica membrane by centrifuging at 11,000 x g for 1 min.
- Add 15 - 30 µl NE buffer and allow it to sit at RT for 1 min. Then, centrifuge at 11,000 x g for 1 min. Remove the silica gel and keep the contents in the tube.
- Use a sequencing kit to determine the sequence according to the manufacturer's protocol.
3. Intramuscular Injections or Open Muscle Biopsy
- For maintenance of sterile conditions during survival surgery, remove hair using clippers in the area surrounding the surgery site (the hind limb). Use iodophors or chlorohexidine as a disinfectant. Use sterile drapes for the surgical site by placing and securing them over the entire animal and the operating table.
- Wear clean scrubs, face masks, head covering, sterile gloves, and special shoes for the operating room52,53.
- Inject CXMD dogs with 20 mg/kg of thiopental sodium to anesthetize them. Use vet ointment on the eyes to prevent the eyes from drying out.
- Use 2 - 3% isoflurane inhalation to maintain general anesthesia (Figure 3). Check muscle reflexes and monitor heart and breathing rate to assess the depth of anesthesia. The normal respiration rate (RR), heart rate (HR), and SpO2 under general anesthesia are as follows: RR: 10 - 20 breaths/min; SpO2: 95 - 100%, HR: 80 - 120 beats per minute (bpm).
- Using a scalpel, cut the skin over the cranial tibial (CT), also known as the tibialis anterior (TA), and make a single cut approximately 5 cm longitudinally (for young adult dogs). To mark the injection sites, stitch the deep fascia using a surgical needle and surgical thread and make two stitch markers at 2 cm intervals.
- Inject the desired concentration of AONs into the muscle with a 27 G needle. The desired concentration varies between treatment conditions. For CT, give two injections of 1 ml volume each, for a total of 1.2 mg of cocktail PMOs (0.4 mg each PMO) or 0.4 mg of cocktail vPMOs (0.13 mg each vPMO). For the extensor carpi ulnaris (ECU) forelimb muscle, inject two volumes of 0.5 ml each for a total of 1.2 mg of cocktail PMOs (0.4 mg each PMO) or 0.4 mg of cocktail vPMOs (0.13 mg each vPMO). Leave the needle in for 1 min. Use an equal amount of each AON for the cocktail.
- Perform open muscle biopsy by removing a piece of muscle tissue approximately 2 cm in length from CT muscle using a surgical scalpel.
- Go to step 6.5 for muscle sample preparation.
- Using a needle, administer an intramuscular injection of 0.02 mg/kg buprenorphine hydrochloride before awakening from general anesthesia. Lay muscle fascia and skin back over muscle and stitch these using a 3-0 absorbable thread and 3-0 nylon thread for muscle fascia and skin closure, respectively.
- While holding the mouth open, determine whether the gag reflex has returned; when the gag reflex has returned, extubate the dog.
- Administer 15 to 30 mg/kg cefazolin or cephalexin (antibiotics) for up to 3 days via intravenous or intramuscular injection to prevent infection.
- Remove sutures within seven days. Do not leave the dog unattended until it has regained sufficient consciousness to maintain sternal recumbency and do not allow the dog to interact with other animals until a full recovery is made. Keep the endotracheal tubes in place as long as possible and remove them when the animal begins to chew or swallow. Monitor the animal's heart rate, respiration, and hydration to make sure they are stable and within normal limits.
- For post-surgery care, provide analgesia for 3 days (e.g., buprenorphine 0.01 mg/kg) and nursing support, including a quiet, dark resting place, appropriate wound and bandage maintenance, a soft resting surface, rehydration with oral or parenteral fluids, and a return to normal feeding through the use of highly palatable foods or treats. If any animals require the additional medication, give an intramuscular injection of 0.3 mg/kg of butorphanol tartrate depending on the pain symptoms.
Note: Dogs in pain may bite, scratch, or guard painful regions, and if handled, may be unusually apprehensive or aggressive. In addition, pain in one limb usually results in limping or holding-up of the affected limb with attempts to use it. In such situations, give an intramuscular injection of 0.02 mg/kg of buprenorphine hydrochloride every 6 - 8 hr.
4. Systemic Injections
Note: This procedure can be repeated weekly or biweekly for the desired number of weeks.
- Restrain dogs manually and gently by getting the dog to lie down and then drape arms over the shoulders and hips to hold the dog in place throughout the procedure.
- Inject AONs; 120 - 240 mg/kg cocktail PMOs (40 - 80 mg each AON) using a venous indwelling needle into a limb vein (known as a cephalic or a saphenous vein). The amount of AON injected depends on the experimental condition. Use an equal amount of each AON for the cocktail.
- Use an infusion pump or syringe driver to inject 50 ml total at a rate of 2.5 ml/min for 20 min, following the manufacturer's instructions. Repeat injections weekly or biweekly (every two weeks) at least 5 times, as dystrophin expression will accumulate with repeated injections.
- Perform blood tests weekly to examine toxicity.
- Using a needle, collect 3 ml of blood — 0.5 ml for complete blood count (CBC) and 2.5 ml for others — from one of the subcutaneous veins of the fore or hindlimb. Include CBC, gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), alanine aminotransferase (ALT), creatine kinase (CK), and creatinine assessments when testing the collected blood, following manufacturer's instructions from blood test kits. 40,54
5. Clinical Grading of Dogs
- Set up a video camera and record dog behavior and gait. Record the entire encounter with the dog as this will act as a reference when grading the dog; this means each video will be a different length depending on the dog's abilities and willingness. Use default recording parameters. Filming creates a record of the grading so it can be reviewed at a later date.
- Grade gait and movement disturbances.
- For gait and movement disturbances, use the following grades:
grade 1 = none, grade 2 = sitting with hind limb extended, grade 3 = bunny-hops with hind limbs, grade 4 = shuffling walk, and grade 5 = unable to walk. - For mobility disturbance, use the following grades: grade 1 = none, grade 2 = lying down more than normal, grade 3 = cannot jump on hind limbs, grade 4 = increasing difficulty moving around, and grade 5 = unable to get up and move around.
- For gait and movement disturbances, use the following grades:
- Time how long it takes the dog to run 15 m. Measure out 15 m, place the dog at the starting line and encourage it to run until the 15 m mark.
- Determine muscle atrophy of the limb using the following grading scale:
grade 1 = none, grade 2 = suspect hardness, grade 3 = can feel hardness or appears thin, grade 4 = between grades 3 and 5, and grade 5 = extremely thin or hard. - Grade drooling using the following scale: grade 1 = none, grade 2 = occasionally dribbles saliva when sitting, grade 3 = some drool when eating and drinking, grade 4 = strings of drool when eating or drinking, and grade 5 = continuous drool.
- Grade hypertrophy of the tongue (macroglossia) using the following scale: grade 1 = none, grade 2 = slightly enlarged, grade 3 = extended outside dentition, grade 4 = enlarged and slightly thickened, and grade 5 = enlarged and thickened.
- Grade the dog's ability to swallow using the following scale: grade 1 = no difficulty, grade 2 = takes time and effort in taking food, grade 3 = difficulty in taking food from a plate, grade 4 = difficulty in chewing, swallowing, or drinking, and grade 5 = unable to eat.
- Calculate total grade by adding the scores from each category (except for the 15 m running test) 55.
Note: The studies should be blinded so as not to introduce bias.
6. Magnetic Resonance Imaging (MRI)
- Use a 3 Tesla (3T) MRI and 18 cm diameter/18 cm length human extremity coil to obtain T2-weighted images of hind-limbs.
- Anesthetize animals by injecting CXMD dogs with 20 mg/kg of thiopental. Use vet ointment on the eyes to prevent the eyes from drying out. Use 2-3% isoflurane inhalation to maintain general anesthesia.
- Check muscle reflexes and monitor heart and breathing rate to assess the depth of anesthesia. The normal respiration rate (RR), heart rate (HR) and SpO2 under general anesthesia are as follows: RR: 10 - 20 breaths/min, SpO2: 95 - 100%, HR: 80 - 120 beats per minute (bpm).
- Use the following settings to obtain T2-weighted images: TR/TE = 4,000/85 msec, slice thickness = 6 mm, slice gap = 0 mm, field of view = 18 cm x 18 cm, matrix size = 256 x 256, and number of acquisitions = 3 during fast spin echo (Figure 4).
7. Muscle Sampling and Preparation (Necropsy)
Note: Muscles should be sampled one or two weeks after the last AON injection.
- Inject dogs with 20 mg/kg of thiopental sodium to anesthetize them. Use vet ointment on the eyes during anesthetization to prevent drying of the eyes.
- Use 2 - 3% isoflurane inhalation to maintain anesthesia. Check muscle reflexes and monitor heart and breathing rate to assess the depth of anesthesia. The normal respiration rate (RR), heart rate (HR) and SpO2 under general anesthesia are as follows: RR: 10 - 20 breaths/min; SpO2: 95 - 100%, HR: 80 - 120 bpm.
- Euthanize dogs by exsanguination under general anesthesia (for necropsy only). Use exsanguination under deep general anesthesia to avoid the effects caused by blood-derived factors in the molecular analysis (e.g., cardiac muscles).
- Dissect the following muscles (Figure 5): CT, extensor digitorum longus (EDL), gastrocnemius, soleus, biceps femoris, rectus femoris, biceps brachii, triceps brachii, deltoid, ECU, extensor carpi radialis (ECR), flexor carpi ulnaris (FCU), flexor carpi radialis (FCR), gracilis, intercostal, abdominal muscles, diaphragm, lateral dorsi, esophagus, sternocleidomastoid, and heart. To examine toxicity, collect kidney and liver samples. Use Evans and Alexander de Lahunta (2009) as a reference for dissection technique 56.
- Cut the CT, EDL, gastrocnemius, soleus, biceps femoris, rectus femoris, biceps brachii, triceps brachii, deltoid, ECU, ECR, FCU, FCR, gracilis, intercostal, abdominal muscles, lateral dorsi, esophagus, sternocleidomastoid, heart, kidney, and liver into small sections approximately 1 - 1.5 cm in length. Roll the diaphragm up and then cut it into 1 - 1.5 cm sections 56.
- Place tragacanth gum so that it is approximately 0.5 - 1 cm thick on cork discs. Label discs with the animal's identification and muscle name on the opposite side of the tragacanth gum.
- Place muscles with their longitudinal axis perpendicular to the cork in the tragacanth gum.
- Place the corks in a container of isopentane that is sitting in liquid nitrogen. Move it around constantly with tweezers for 1 min or until completely frozen. Store muscles on corks in vials at -80 °C. When transporting, put vials on dry ice.
- Prepare glass slides labeled with animal identification/muscle name and date.
- In a cryostat cooled to -25 °C, mount the cork to the stand. Set the section thickness to the desired level. Use 8 µm for immunohistochemistry and 12 µm for hematoxylin and eosin (HE) staining. Use 15 µm for Western blot samples. Trim about one-quarter of the muscle to obtain a flat muscle for proper muscle sampling.
- Cutting one section of muscle at a time, place every 6th section on the same glass slide if planning to use samples for immunohistochemistry or HE staining. If using samples for Western blots, put 30 - 40 sections in a tube and store at -80 °C.
- After preparation of slides, let slides dry for 1.5 hr at RT. Store slides at -80 °C.
8. Immunohistochemistry
- Remove prepared slides from storage and place them in a moisture chamber to keep the slides separated and maintain moisture. Fill the moisture chamber so that the bottom is just covered with water (approximately 1 mm of water).
- Air-dry for 0.5 hr. Draw a square enclosing the muscle sample on the slide using a hydrophobic barrier pen.
- Add 15% goat serum in phosphate buffered saline (PBS) and incubate for 2 hr at RT In blocking reagent.
- Add anti-dystrophin rod domain (DYS-1) mouse monoclonal primary antibody, or C-terminal monoclonal antibody (DYS-2) for dog dystrophin staining (1:150 dilution). Dilute using a blocking agent in 1.25 ml of PBS. Incubate O/N in a refrigerator a 4 °C.
- Wash with PBS for 5 min, repeated three times. Add secondary antibody Alexa 594 goat antibody against mouse IgG1 (for DYS-2) or IgG2 (for DYS-1) (1:2,500). Dilute with a blocking reagent in PBS containing 0.1% octyl phenol ethoxylate. Incubate for 0.5 hr at RT.
- Wash with PBS for 5 min, three times. Allow slide to dry. Add 1 - 2 drops (3 ng/ml) of 4',6-diamidino-2-phenylindole (DAPI) mounting solution. Using tweezers, place a glass slip over the top of the sections, avoiding bubbles.
- View dystrophin- (DYS-1/DYS-2) positive fibers under a fluorescent microscope at 594 nm at 20X magnification.
9. Western Blotting
- Add collected muscle sections from cryo-sectioning to 150 µl of sample buffer on ice.
- Using a hand homogenizer, briefly homogenize the protein samples. Heat samples in a 1.5 ml tube in a heating block incubator at 95 °C for 3 - 5 min. Centrifuge at 16,500 × g for 15 min and collect the supernatant.
- Store aliquots of the supernatant at -70 °C. Use distilled water to dilute to 100x.
- Determine protein concentration using a commercial kit according to manufacturer's protocol. Add 2x Laemmli SDS-loading buffer to samples and heat for 3 min at 95 °C.
- Load samples into 3 - 8% Tris-acetate gel before running it for 3 hr at 150 V. Include several diluted wild-type (WT) samples (e.g., 1%, 10%, and 50%) for quantification. Less than 1% WT levels of dystrophin should be detected with this method.
- Place the gel in cathode buffer for 20 min. During this time, take nine pieces of filter paper and put them in the transfer buffers (3 papers in the cathode buffer [+], the anode buffer ([-], and the concentrated anode buffer [--], respectively). This describes a semi-dry transfer method which increases sensitivity (Figure 6).
- Soak a PVDF membrane in methanol for 20 sec and then place in the anode buffer.
Set up the gel with the membrane for semi-dry transfer and allow it to run 1.5 hr at 400 mA in RT or in a cold room. - Wash the membrane with PBS. Incubate the membrane in a mixture of PBS with Tween 20 (PBST) and 5% milk powder for 2 hr.
- Dilute DYS-1 (primary antibody) in PBST and 5% milk powder mixture to a 1:100 dilution. Add it to the membrane and let it incubate for at least 1 hr. Wash three times with 100 ml PBST for 15 min each.
- Add 65 µl/lane of HRP conjugated secondary antibody (anti-mouse IgG2a for DYS1) at 1:5,000 dilution for 1 hr at RT in a dark area. Then, wash with three times with 200 ml PBST for 20 min. Mix solutions from a detection kit as directed. Incubate for 1 min.
- Develop film to detect bands and analyze with ImageJ software 48,57,58.
Wyniki
In Vitro Experiments
Myoblasts were transfected with various 2'OMePS treatment conditions in order to compare the effectiveness of each AON. Single AON treatments with 600 nM each of Ex6A, Ex6B, Ex8A, or Ex8B were done, as well as a cocktail treatment with 600 nM each of all 4 AON sequences. RNA samples were collected four days after transfection. After RT-PCR, samples for each treatment were run on a gel along with non-treated (NT) samples. Bands higher on the gel represent out-of-frame DMD products; these bands were seen in NT, Ex8A, and Ex8B treated myoblasts. Ex6A, Ex6B, Ex8A, and the cocktail-treated myoblasts showed in-frame products. The cocktail and Ex6A/B showed 100% in-frame products, while Ex8A showed only 30% in-frame products (Figure 7). To confirm exon skipping and restoration of the reading frame, cDNA sequencing was performed; the results indicated that exons 6 - 9 had indeed been skipped (Figure 7). Immunohistochemistry showed that AON-treated dogs had increased dystrophin-positive fibers compared to NT samples (Figure 8).
In Vivo Experiments
To compare the efficiency of various AON treatment conditions, CXMD dogs (0.5 - 5 years old) were injected once with 1.2 mg Ex6A or a cocktail of Ex6A, Ex6B, and Ex8A at various dosages. Two weeks after the injection, muscle samples were collected and stained with DYS-1 to compare the number of dystrophin-positive fibers. All cocktail-treated samples showed increased dystrophin expression compared to NT samples. Dystrophin-positive fibers increased with AON dosage (Figure 9). Following systemic injection, wild-type (WT), NT, and cocktail-treated CXMD muscle samples were stained with DYS-1 (Figure 10). Cocktail-treated CXMD dogs showed increased dystrophin expression compared to NT CXMD dogs, both in CT and heart muscle samples. However, AON-treated skeletal muscle (CT) showed much higher expression of dystrophin compared to treated cardiac muscle. An immunoblot comparing WT, NT, and various morpholino cocktail-treated muscles led to the same conclusion. There was also a large range of dystrophin expression in the treated skeletal muscle samples (Figure 10). Hematoxylin and eosin (HE) staining revealed that treated CXMD dogs showed improved histopathology, with a significant decrease in centrally-nucleated fibers (CNF) in comparison to NT CXMD dogs (Figure 11). This indicates there is more degeneration/regeneration occurring in the NT dog, a sign of dystrophic muscle pathology. Additionally, treated dogs had faster running times and improved scores on the clinical grading scale. Treated CXMD dogs showed better scores than NT CXMD dogs in all categories (Figure 12).
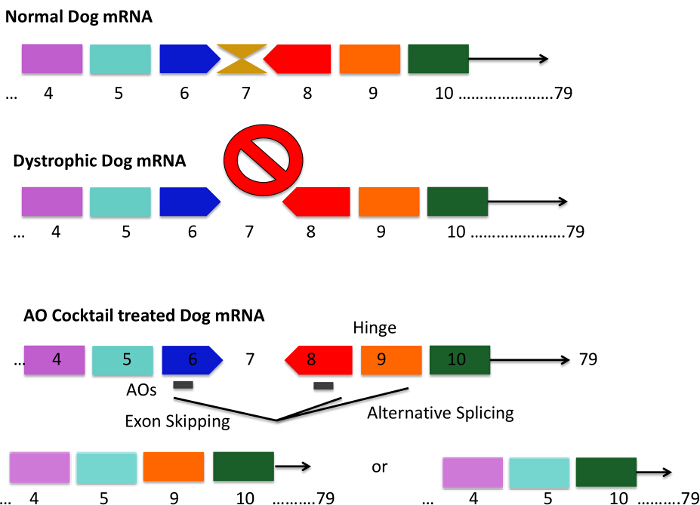
Figure 1. Mutation Pattern of the CXMD Dog and Exons 6 - 8 Skipping Strategies Using an Antisense Cocktail. CXMD dogs have a point mutation in exon 6 leading to a loss of exon 7 in dystrophic dog mRNA. This results in the mRNA being out-of-frame and dystrophin protein production is lost. Short AON sequences are designed to bind to exon 6 and 8, which results in mRNA splicing effectively skipping exons 6 - 8. The gray bar in the AON cocktail-treated dogs represents short AON sequences. Exon 9 encodes a hinge domain and is sometimes spontaneously spliced out with AONs against exon 6 and 8. The resulting mRNA codes for dystrophin proteins that are shorter but functional. Please click here to view a larger version of this figure.
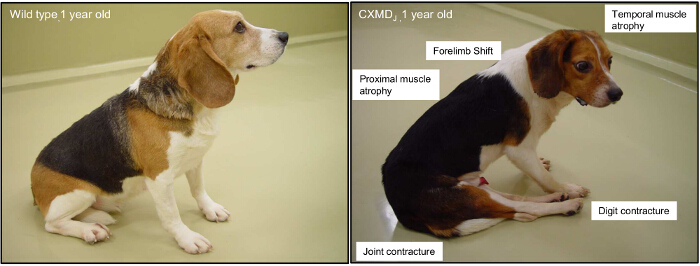
Figure 2. Major Clinical Symptoms of a 1-year-old Canine X-linked Muscular Dystrophy (CXMD) Animal. A 1-year-old wild-type beagle and a CXMD dog are shown. The involvement of proximal, limb, and temporal muscles are typically observed from 2 months of age. Joint contracture and a shifting of the pelvis are overt from 4 months of age. Please click here to view a larger version of this figure.
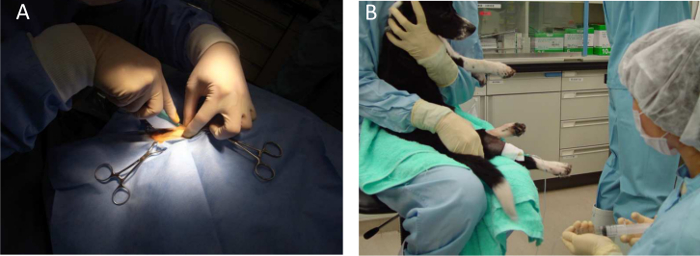
Figure 3. General Anesthesia for a Dog. A) Intramuscular injections and muscle biopsies are performed under general anesthesia with isoflurane. B) Holding of the animal for systemic injections. Please click here to view a larger version of this figure.
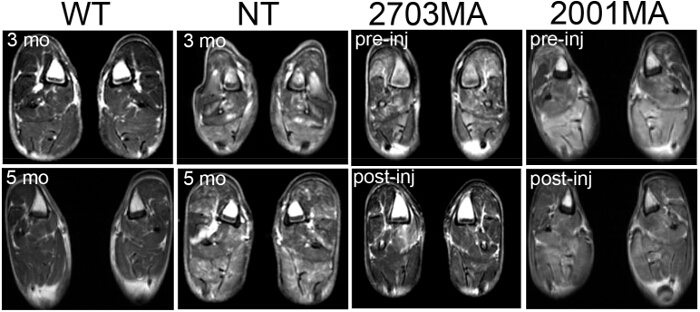
Figure 4. Magnetic Resonance Imaging (MRI) of Wild-type, Non-treated CXMD, and Treated CXMD. MRI scans of the hind limb at 3 months and 5 months in WT and NT CXMD dogs. Two sample images of treated CXMD hind limb MRIs pre- (1 week before the first injection) and post-injection of AON are shown. 2703MA was treated 7x weekly with 200 mg/kg cocktail morpholinos. 2001MA was treated with 5x weekly IV injection of 120 mg/kg cocktail morpholinos. Control and treated dogs were age-matched. Treated dogs show decreased T2 signals. Images are adapted with permission from Yokota et al. (copyright 2009, John Wiley & Sons) 40 Please click here to view a larger version of this figure.
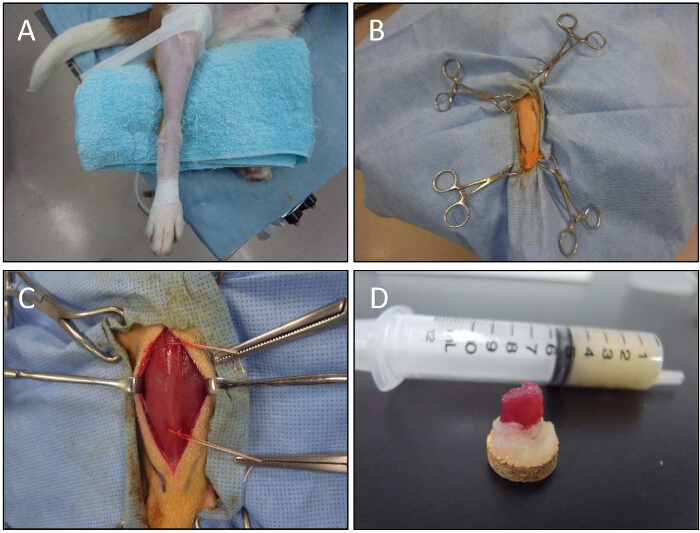
Figure 5. Muscle Biopsy Procedure for a Dog. A) A lower limb is fixed for muscle biopsy. B) With the help of forceps, the lower limb is held. C) The CT muscle is exposed. Open biopsy technique is used to obtain muscle samples of injected sites. Threads are used to hold biopsy samples. D) Muscle samples on tragacanth gum after dissection. Please click here to view a larger version of this figure.

Figure 6. Semi-dry Transfer Method. A representation of the semi-dry transfer method for Western blotting is presented. Three papers soaked in concentrated anode buffer are laid down at the negative terminal; 3 papers soaked in anode buffer are stacked on top of this. The Mb PVDF paper is soaked in methanol and then anode buffer before being laid on top of the 6 papers. The gel, which has been soaked in cathode buffer, is laid gently over the PVDF paper. Finally, 3 papers soaked in cathode buffer are laid on top of the gel. The positive terminal is set on top. For 1 hr, 400 mA is run through the system. Please click here to view a larger version of this figure.
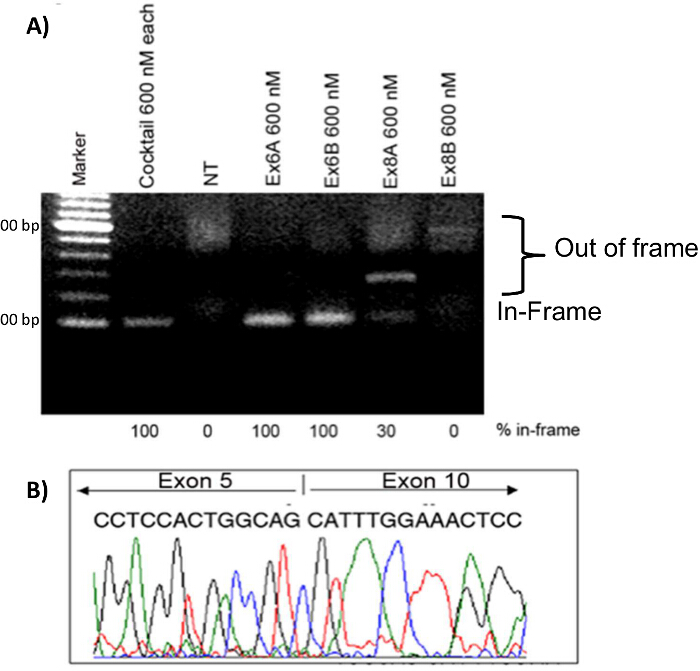
Figure 7. Exon Skipping in CXMD Myoblasts. CXMD myoblasts were transfected with Ex6A, Ex6B, Ex8A, or Ex8B alone, or a cocktail of all four. A total of 600 nM was used for the individual sequences and for the cocktail 600 nM of each sequence was used. A) 2'OMePS treatment in CXMD dog myoblasts. Ex6A, Ex6B, and the cocktail-treated samples show strong bands at the expected position of in-frame exon-skipped transcripts. Ex8A shows an intermediate band, Ex8B shows a weak band, and NT does not show a band at the in-frame position. B) cDNA sequencing from Ex6A alone, 4 days after transfection. Images are adapted with permission from Yokota et al. (copyright 2009, John Wiley & Sons) 40. Please click here to view a larger version of this figure.

Figure 8. Increased Dystrophin Expression in 2'O-methylated Phosphorothioate (2'OMePS) Transfected CXMD Myoblasts. CXMD myoblasts were transfected with Ex6A alone or with cocktail 2'OMePS. DYS-2 (red) and DAPI (blue) staining are shown. The treated myoblasts are compared to wild-type (WT) and non-treated (NT) myoblasts. Images are adapted with permission from Yokota et al. (copyright 2009, John Wiley & Sons) 40. Bar = 50 µm. Please click here to view a larger version of this figure.
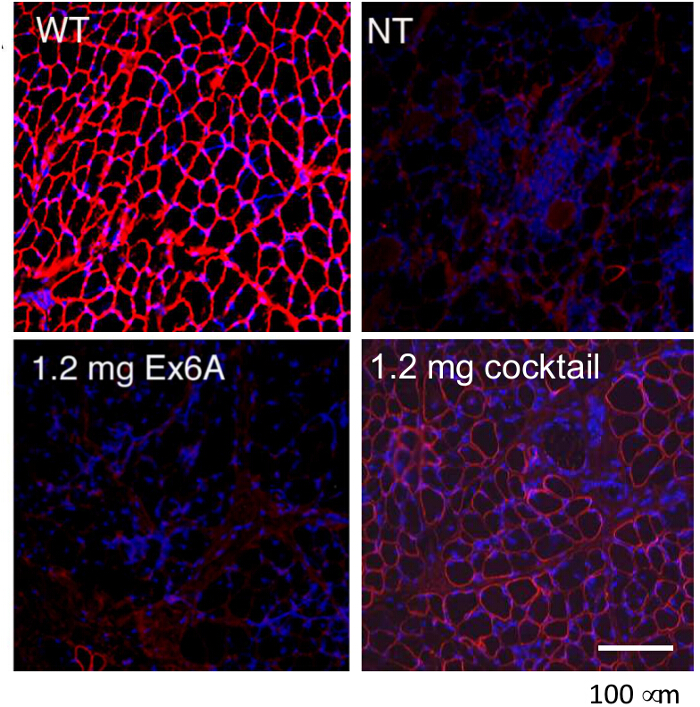
Figure 9. Rescue of Dystrophin Expression with Intramuscular Injections of Morpholinos in CXMD Dogs. Either Ex6A alone or a cocktail of Ex6A, Ex6B, and Ex8A were injected into the CT muscles of CXMD dogs. Dystrophin (DSY-1) staining of wild-type (WT), non-treated (NT), and treated CXMD dogs are shown. Dogs were either treated with 1.2 mg Ex6A alone or 1.2 mg cocktail. Images are adapted with permission from Yokota et al. (copyright 2009, John Wiley & Sons) 40. Bar = 100 µm. Please click here to view a larger version of this figure.

Figure 10. Increased Dystrophin Expression After Systemic Cocktail Morpholino Treatment in CXMD Dogs. Dystrophin (DYS-1) staining was used to compare dystrophin expression in wild type (WT) (positive control), non-treated (NT) (negative control), and CXMD dogs treated with 120 mg/kg morpholino cocktail (40 mg/kg of each AON). The morpholino cocktail contained Ex6A, Ex6B, and Ex8A. Dogs were injected intravenously 5 times weekly with this cocktail. A) A comparison of dystrophin expression in cranial tibial (CT) muscles of WT, NT, and treated dogs. B) A comparison of dystrophin expression in heart tissue between NT and morpholino cocktail-treated dogs. C) Immunoblot for dystrophin with desmin as a loading control is shown for WT, NT, and morpholino cocktail-treated dogs. The following muscles are shown for treated dogs: triceps brachii (TB), biceps brachii (BB), diaphragm (DIA), esophagus (ESO), CT, adductor (ADD), extensor digitorum longus (EDL), masseter (MAS), and heart. Images are adapted with permission from Yokota et al. (copyright 2009, John Wiley & Sons) 40. Bar = 200 µm. Please click here to view a larger version of this figure.
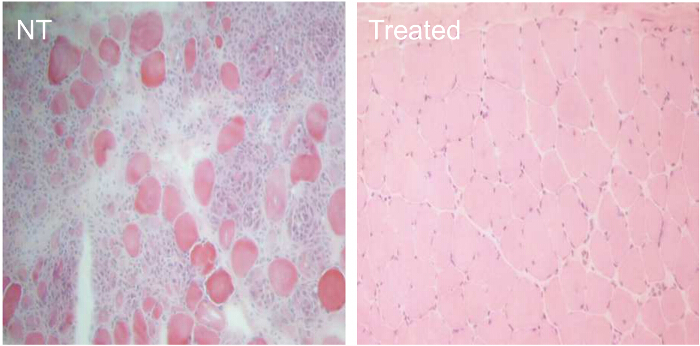
Figure 11. Improved Histopathology in CXMD Dogs Treated For 7 Weeks with 240 mg/kg Morpholino Cocktail. CXMD dogs ranging from half a year to five years old were injected intravenously with 240 mg/kg morpholino cocktail (Ex6A, Ex6B, and Ex8A) once a week for 7 weeks. Fourteen days after the last injection, esophagus muscles were taken and hematoxylin and eosin (HE) staining was done. HE staining of esophagus muscles from non-treated (NT) and morpholino cocktail-treated (Treated) CXMD dogs (40X objective lens). Please click here to view a larger version of this figure.
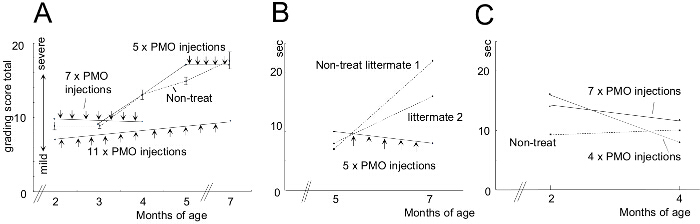
Figure 12. Improved Scores on Clinical Grading and 15 m Running Time After Morpholino Treatment. Morpholino-treated dogs were compared to non-treated (NT) littermates. Error bars in the graph indicate SEM. A) Total score on the clinical grading exam was calculated before and after treatment and treated animals were compared with NT littermates. B) A comparison of 15 m running times of treated and NT dogs. C) Similar to B; however, younger dogs were used. Images are adapted with permission from Yokota et al. (copyright 2009, John Wiley & Sons) 40. Please click here to view a larger version of this figure.
| Antisense Oligonucleotide | Nucleotide Sequence |
| Ex6A | GTTGATTGTCGGACCCAGCTCAGG |
| Ex6B | ACCTATGACTGTGGATGAGAGCGTT |
| Ex8A | CTTCCTGGATGGCTTCAATGCTCAC |
Table 1. Antisense Oligonucleotide Design.
Dyskusje
Exon skipping is a promising therapeutic technique for the treatment of DMD. Both in vitro and in vivo experiments have shown that multi-exon skipping is feasible. Here, the use of the CXMD dog model is discussed. First, AONs were designed using the Rescue-ESE and ESEfinder programs to target dystrophin exons 6 - 8. The 2'OMePS AON chemistry was used for CXMD myoblast transfection and the morpholino AON backbone chemistry was chosen for in vivo experiments. vPMOs are more efficient than unmodified PMOs but due to their higher toxicity they are not suitable for systemic injections. RNA extraction, RT-PCR, and cDNA sequencing were performed on the CXMD myoblasts. Dogs injected with the PMO cocktail were clinically graded to assess any improvement in clinical symptoms. After the dogs were humanely euthanized, muscle samples were taken and prepared for cryo-sectioning. The half-life of dystrophin protein induced by AONs is believed to be approximately 1 - 2 months. Young adult dogs were used in this study, although these experiments can be done with neonatal dogs and older dogs (>5 years old). Prepared muscle sections were used to evaluate histopathology and assess dystrophin protein rescue through Western blotting and immunohistochemistry48.
It is important to ensure that the volume of the PMO solution is correct before injections; failing to do so will have significant effects on results. During intramuscular injections, sufficient pressure is required to enter the muscle fibers. Monitoring of dog health and inspection of the surgery site are important for troubleshooting. To monitor animal health, weekly blood tests and weighing should be performed. After euthanization of the animal and preparation of muscle samples, a critical step for ensuring sensitivity in detecting dystrophin protein is to use both the Tris-acetate gel and semi-dry blotting method during the Western blotting procedure.
As shown in the representative results, myoblasts treated with Ex6A, Ex6B, Ex8A, and the cocktail (containing Ex6A, Ex6B, Ex8A, and Ex8B) produced in-frame DMD products. Since Ex8B produced no exon-skipped products, it was not used in subsequent in vivo experiments. cDNA sequencing showed that exons 6 - 9 skipping occurred and immunocytochemistry with DYS-2 staining showed restored dystrophin expression in treated samples. AON-treated dogs showed a significant increase in dystrophin-positive fibers. This indicates that exons 6 - 8 were being skipped and a shortened protein was produced. The amount of dystrophin-positive fibers increased when a cocktail of AONs was used and was proportional to AON dosage. Immunoblots showed increased dystrophin expression in systemic morpholino-treated dogs. Skeletal muscle had variable levels of dystrophin fibers; however, morpholino-treated heart tissue showed little improvement in dystrophin expression. Since dystrophin has a high molecular weight (427 kDa), detection of low amounts of dystrophin can be difficult. For the best results, Tris-acetate gel and the semi-dry transfer method were used. HE staining showed improved histopathology in the morpholino-treated dogs. Centrally-nucleated fibers (CNFs) are a sign of unhealthy muscle and represent cycles of muscle degeneration and regeneration. Morpholino-treated CXMD dogs showed a decrease in the percentage of CNFs in comparison to non-treated CXMD dogs. Clinical grading revealed an improvement in symptoms, such as increased walking and running ability, in morpholino-treated animals. The hardness of muscles is believed to reflect muscle atrophy, thus, it was included in the grading scheme 59. The hardness of thigh (hind-limb) muscles was evaluated; however, we excluded cranial sartorius muscles because they tend to exhibit hypertrophy rather than atrophy in CXMD. Treated dogs showed lower grading scores and had faster times on the 15 m running test. Improved times on the 15 m test are indicative of improved muscle function 40. Higher overall grading scores indicate poor health and increased muscle atrophy.
While these results are promising, multi-exon skipping still presents many challenges that will need to be overcome before the technique has clinical applicability. Cardiac tissue still displays reduced uptake of AONs, likely due to the difference in cellular trafficking between cardiac and skeletal tissue. No toxic effects have been observed in animals under current dosage regimens; however, more work needs to be done to assess long-term toxicity before the use of AON cocktails can move to clinical trials. It is difficult to gain approval for AON cocktail drugs because regulatory agencies define each AON sequence as a unique drug. This means that each sequence in a cocktail would need to be individually tested for safety, requiring more time and more money. Another barrier to the use of multi-exon skipping in a clinical setting is a large amount of intermediate protein products produced with unknown functions. These proteins can potentially lead to unpredictable side-effects, depending on the individual mutation 22. Additionally, the mutation patterns available within current dystrophic dog models are limited. There are few naturally-occurring mutations, and not all mutations are useful for studying multi-exon skipping. The dystrophic pig model promises to be a good alternative for future DMD exon skipping studies 33, 34.
The DMD dog models have some advantages over other DMD models. Being a larger animal model, clinical grading and MRI are possible, allowing for more detailed analysis. Since dogs are large animals they are also more suited for toxicology studies and more closely represent the human disease in comparison to the mouse model. Dog models also have DMD gene sequences that are more similar to humans 23, 34, 45.
Although technically challenging, the multi-exon skipping approach could ultimately benefit >90% of DMD patients 24. This makes it a much better alternative to single-exon skipping, as single-exon skipping is only applicable to a small subset of patients. In addition, multi-exon skipping will enable us to select the deletion patterns that optimize the functionality of the shortened dystrophin proteins. For example, deletion of DMD exons 45 - 55 is associated with exceptionally mild symptoms or asymptomatic individuals 14,19,60-63. The multi-exon skipping of exons 45 - 55 has already been demonstrated in a mouse model of DMD with an exon 52 deletion (mdx52) using systemic injections of vPMOs 22,26. The use of cocktail vPMOs has also been demonstrated in other forms of muscular dystrophy, such as Fukuyama congenital muscular dystrophy (FCMD). FCMD is caused by exon trapping, in which aberrant mRNA splicing is caused by retrotransposon insertion. vPMOs have been shown to rescue the splicing pattern in both an FCMD mouse model and in human cell lines 64. Next-generation AON chemistries exhibiting higher efficacy and lower toxicity would facilitate the effective translation of the multi-exon skipping approach into clinical application. Additionally, multi-exon skipping could potentially be applied to other genetic disorders, such as the dysferlinopathies 24, 65.
Ujawnienia
Open access fees for this article were provided by, Gene Tools, LLC.
Podziękowania
This work was supported by The University of Alberta Faculty of Medicine and Dentistry, The Friends of Garrett Cumming Research Chair Fund, HM Toupin Neurological Science Research Chair Fund, Muscular Dystrophy Canada, Canada Foundation for Innovation (CFI), Alberta Advanced Education and Technology (AET), Canadian Institutes of Health Research (CIHR), Jesse's Journey - The Foundation for Gene and Cell Therapy, and the Women and Children's Health Research Institute (WCHRI).
Materiały
| Name | Company | Catalog Number | Comments |
| 2'OMePS Transfection of Dog Myoblasts | |||
| 3 ml 6-well plates | IWAKI | 5816-006 | |
| Dulbecco’s modified Eagle’s medium (DMEM) | Gibco | 11965-092 | |
| Fetal bovine serum (FBS) | HyClone | SH30071.01 | |
| Penicillin | Sigma-Aldrich | P4333 | 200 U/ml |
| Lipofectin | Invitrogen | 18292-011 | Total volume of 100 ml in opti-MEM media at a ratio of 2:1 for lipofectin. 10 ml lipofectin for 5 mg RNA. |
| 2’OMePS | Eurogentec | Ex6A (GUU GAUUGUCGGACCCAGCUCAGG), Ex6B (ACCUAUGA CUGUGGAUGAGAGCGUU), and Ex8A (CUUCCUGG AUGGCUUCAAUGCUCAC). | |
| Horse Serum | Gibco | 16050-114 | 2% |
| streptomycin | Sigma-Aldrich | P4333 | 200 μg/ml |
| Bovine serum albumin (BSA) | Sigma-Aldrich | A9418 | |
| Insulin | |||
| Morpholino transfection of dog myoblasts All material from MePS Transfection of Dog Myoblasts for culturing | |||
| Antisense morpholinos | Gene-tools | Ex6A(GTTGATTGTCGGACCCAGC TCAGG), Ex6B(ACCTATGACTGTGGATGAG AGCGTT), Ex8A(CTTCCTGGATGGCTTCAAT GCTCAC) Dilute to a final volume of 100 L in opti-MEM media. 120–200 mg/kg of morpholinos at 32 mg/ml in saline | |
| Endo-Porter | Gene-tools | ||
| guanidinium thiocyanate-phenol-chloroform | Invitrogen | 15596-018 | 1 ml/plate |
| RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR) | |||
| guanidinium thiocyanate-phenol-chloroform | Invitrogen | 15596-018 | 1 ml/plate |
| Chloroform | Sigma-Aldrich | P3803 | 200 μl |
| 1.5 ml Tubes | Eppendorf | 22363204 | |
| Centrifuge | Beckman-Coulter | ||
| 75% Ethanol | Sigma-Aldrich | 34852 | |
| UV Spectrometer | |||
| Forward primer in exon 5 | Invitrogen | CTGACTCTTGGTTTGATTTGGA 1.5 μl 10 μM | |
| Reverse primer in exon 10 | Invitrogen | TGCTTCGGTCTCTGTCAATG 1.5 μl 10 μM | |
| dNTPs | Clontech | 3040 | |
| One-Step RT-PCR kit | Qiagen | 210210 | |
| Thermo-cycler | Scinco | ||
| Complementary DNA (cDNA) Sequencing | |||
| Gel extraction kit | Qiagen | 28704 | |
| Centrifuge | |||
| Terminator v3.1 Cycle Sequencing Kit | Applied Biosystems | 4337454 | |
| Intramuscular injections or open muscle biopsy | |||
| Surgical Tools | Scissors, scalpel, needle, surgical thread | ||
| Vet Ointment | |||
| Iodophors | webtextiles | 12190-71-5 | |
| chlorohexidine | Peridex | 12134 | |
| Surgical Drapes | |||
| Scrubs | |||
| Facial Mask | |||
| Surgical Gloves | |||
| Head Covering | |||
| Thiopental sodium | Mitsubishi Tanabe Pharma | 20 mg/kg | |
| Isoflurane | Abbott laboratories | 05260-05 | 2-3% |
| Antisense morpholinos | Gene-tools | Ex6A(GTTGATTGTCGGACCCAGC TCAGG), Ex6B(ACCTATGACTGTGGATGAG AGCGTT), Ex8A(CTTCCTGGATGGCTTCAAT GCTCAC) Dilute to a final volume of 100 L in opti-MEM media. 120–200 mg/kg of morpholinos at 32 mg/ml in saline | |
| 27 G Needles | TERUMO | SG3-2325 | |
| 50 ml syringe | TERUMO | SG2-03L2225 | |
| buprenorphine hydrochloride | |||
| Tongue Depressor | |||
| cephalexin | 15 to 30 mg/kg | ||
| cefazolin | 15 to 30 mg/kg | ||
| buprenorphine | 0.01 mg/kg | ||
| buprenorphine hydrochloride | 0.02 mg/kg | ||
| Systemic Injections | |||
| Syringe infusion pump | Muromachi | ||
| 22 G Indwelling needles | TERUMO | SG3-2225 | |
| 27 G Needles | TERUMO | SG3-2325 | |
| 50 ml syringe | TERUMO | SG2-03L2225 | |
| Saline | Ohtsuka-Pharmaceutical | 28372 | |
| Clinical Grading of Dogs | |||
| Video Camera | |||
| Stop watch | |||
| Magnetic resonance imaging (MRI) | |||
| 3 Tesla MRI l | |||
| 18 cm diameter/18 cm length human extremity coil | |||
| Muscle sampling and preparation (necropsy) | |||
| Thiopental sodium | Mitsubishi Tanabe Pharma | 20 mg/kg | |
| Isoflurane | Abbott laboratories | 05260-05 | 2-3% |
| Tragacanth gum | 10-20 ml | ||
| Liquid Nitrogen | |||
| Cork Discs | Iwai-kagaku | 101412-806 | |
| Dry Ice | |||
| Tweezers | |||
| Poly-L-lysine–coated slides | Fisher | 22-037-216 | |
| Cryostat Microsystem | Leica | cm1900 | |
| Immunohistochemistry | |||
| DYS1 | Novocastra | NCL-DYS1 | 1:150 dilutions |
| DYS2 | Novocastra | NCL-DYS2 | 1:150 dilutions |
| Alexa 594 goat antimouse IgG1 | Invitrogen | A-21125 | 1:2,500 dilutions |
| Alexa 594 goat antimouse IgG2 | Invitrogen | A-11005 | 1:2,500 dilutions |
| DAPI | Invitrogen | D1306 | Contains mounting agent |
| Goat Serum | Invitrogen | 10000C | 15% |
| PBS | |||
| Moisture chamber | Scientific Devise Laboratory | 197-BL | |
| Chamber slide | Lab-tek | 154453 | |
| Cover Glasses | Fisher | 12-540A | |
| Hydrophobic barrier pen | |||
| Fluorescent microscope | 594 nm at 20X magnification. | ||
| Western Blotting | |||
| Distillied Water | |||
| Hand Homogenizer | |||
| 2× Laemmli SDS-loading buffer | 0.1 M Tris–HCl (pH 6.6), 2% (w/v) SDS, 2% (0.28 M) beta-mercaptoethanol, 20% glycerol, 0.01% bromophenol blue | ||
| SDS gels | Bio-Rad | 161-1210 | 5% resolving |
| SDS gels | Invitrogen | Invitrogen, WG1601BOX | 3-8% |
| PVDF membrane | GE | 10600021 | |
| Methanol | |||
| Running buffer (10×) | 250 mM of Tris-Base, 1,920 mM of Glycine | ||
| Running buffer (1×) | 10% 10× buffer, 20% methanol | ||
| 0.05% PBS/Tween 20 (PBST) | 2,000 ml 3× 200 ml for washing | ||
| PBST/5% milk powder | 100 ml | ||
| Protein Assay Kit | BCA | T9650 | |
| Tween 20 | Sigma | P5927 | |
| Urea | Sigma | U5378 | |
| Beta Mercaptoethanol | Millipore | ES-007-E | |
| SDS | Sigma | L3771 | |
| Tris-Acetate | Sigma | ||
| Tris HCl | Sigma | T3253 | |
| Glycerol | Sigma | G8773 | |
| Loading/sample buffer for Western blotting | NuPage Invitrogen | NP007 | |
| NaCl | Sigma | S3014 | |
| PMSF | Sigma | P7626 | |
| Protease cocktail inhibitor | Roche | 11836153001 | |
| Cathode Buffer | 0.025 M Tris base + 40 mM 6-aminocaproic acid + 20% Methanol | ||
| Anode Buffer | 0.03 M Tris Base + 20% Methanol | ||
| Concentred Anode Buffer | 0.3 M Tris base + 20% Methanol | ||
| desmin antibody | Abcam | ab8592 | |
| DYS1 | Novocastra | NCL-DYS1 | 1:150 dilutions |
| ImageJ Software | |||
Odniesienia
- Duchenne, A. The Pathology of Paralysis with Muscular Degeneration (Paralysie Myosclerotique), or Paralysis with Apparent Hypertrophy. Br Med J. 14 (2), 541-542 (1867).
- Zellweger, H., Antonik, A. Newborn screening for Duchenne muscular dystrophy. Pediatrics. 55 (1), 30-34 (1975).
- Echigoya, Y., Yokota, T. Skipping multiple exons of dystrophin transcripts using cocktail antisense oligonucleotides. Nucleic Acid Ther. 24, 57-68 (2014).
- Bushby, R. F., Birnkrant, D. J., et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. The Lancet Neurology. 9 (1), 77-93 (2010).
- Eagle, M., Baudouin, S. V., Chandler, C., Giddings, D. R., Bullock, R., Bushby, K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul. Disord. 12 (10), 926-929 (2002).
- Heald, A., Anderson, L. V., Bushby, K. M., Shaw, P. J. Becker muscular dystrophy with onset after 60 years. Neurology. 44 (12), 2388-2390 (1994).
- Stöllberger, C., Finsterer, J. Worsening of heart failure in Becker muscular dystrophy after non-steroidal anti-inflammatory drugs. Med. J. 98 (4), 478-480 (2005).
- Passamano, L., et al. Improvement of survival in Duchenne Muscular Dystrophy: retrospective analysis of 835 patients. Acta Myol. 31 (2), 121-125 (2012).
- Hoffman, E. P., Brown, R. H., Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 51 (6), 919-928 (1987).
- Koenig, M., et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 50 (3), 509-517 (1987).
- Takeshima, Y., et al. Mutation spectrum of the dystrophin gene in 442 Duchenne/Becker muscular dystrophy cases from one Japanese referral. JHG. 55 (6), 379-388 (2010).
- White, S. J., et al. Duplications in the DMD gene. Hum. Mutat. 27, 938-945 (2006).
- Yokota, T., Duddy, W., Echigoya, Y., Kolski, H. Exon skipping for nonsense mutations in Duchenne muscular dystrophy: too many mutations, too few patients. Expert Opin. Biol. Ther. 12 (9), 1141-1152 (2012).
- Yokota, T., Duddy, W., Partridge, T. Optimizing exon skipping therapies for DMD. Acta Myol. 26 (3), 179-184 (2007).
- Magri, F., et al. Genotype and phenotype characterization in a large dystrophinopathic cohort with extended follow-up. J Neurol. 258 (9), 1610-1623 (2011).
- Yoshida, H. H., Ishikawa-Sakurai, M. Biochemical evidence for association of dystrobrevin with the sarcoglycan- sarcospan complex as a basis for understanding sarcogly- canopathy. Hum. Mol. Genet. 9 (7), 1033-1040 (2000).
- Bies, R. D., Caskey, C. T., Fenwick, R. An intact cysteine-rich domain is required for dystrophin function. J. Clin. Invest. 90 (2), 666-672 (1992).
- Monaco, A. P., Bertelson, C. J., Liechti-Gallati, S., Moser, H., Kunkel, L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 2 (1), 90-95 (1988).
- Aoki, Y., Yokota, T., Wood, M. J. Development of multiexon skipping antisense oligonucleotide therapy for Duchenne muscular dystrophy. Biomed Res Int. 2013 (402369), (2013).
- Cirak, V. A. -. G., Guglieri, M., et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. The Lancet. 378 (9791), 595-605 (2011).
- Goemans, N. M., et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med. 364 (16), 1513-1522 (2011).
- Echigoya, Y., et al. Long-term efficacy of systemic multiexon skipping targeting dystrophin exons 45-55 with a cocktail of vivo-morpholinos in mdx52 mice. Mol Ther Nucleic Acids. 4, (2015).
- Hoffman, E. P., et al. Restoring dystrophin expression in Duchenne muscular dystrophy muscle progress in exon skipping and stop codon read through. Am J Pathol. 179 (1), 12-22 (2011).
- Aartsma-Rus, A., et al. Antisense-induced multiexon skipping for Duchenne muscular dystrophy makes more sense. Am J Hum Genet. 74 (1), 83-92 (2004).
- Aartsma-Rus, A., Kaman, W. E., Weij, R., Tden Dunnen, J., van Ommen, G. J., van Deutekom, J. C. Exploring the frontiers of therapeutic exon skipping for Duchenne muscular dystrophy by double targeting within one or multiple exons. Mol. Ther. 14 (3), 401-407 (2006).
- Aoki, Y., et al. Bodywide skipping of exons 45-55 in dystrophic mdx52 mice by systemic antisense delivery. Proc Natl Acad Sci U S A. 109 (34), 13763-13768 (2012).
- McClorey, G., Iversen, P. L., Moulton , H. M., Fletcher, S., Wilton, S. D. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther. 13 (19), 1373-1381 (2006).
- Sharp, N. J., et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 13 (1), 115-121 (1992).
- Shimatsu, Y., et al. Canine X-linked muscular dystrophy in Japan (CXMDJ). Exp Anim. 52 (2), 93-97 (2003).
- Nguyen, F., Cherel, Y., Guigand, L., Goubault Leroux, I., Wyers, M. Muscle lesions associated with dystrophin deficiency in neonatal golden retriever puppies. J Comp Pathol. 126 (2-3), 100-108 (2002).
- Nakamura, A., Takeda, S. Mammalian models of Duchenne Muscular Dystrophy: pathological characteristics and therapeutic applications. J Biomed Biotechnol. 2011 (184393), (2011).
- Yokota, T., et al. A renaissance for anti-sense oligonucleotide drugs in neurology: Exon-skipping breaks new ground. Arch. Neurol. 66, 32-38 (2009).
- Aartsma-Rus, A., et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum. Mutat. 30 (3), 293-299 (2009).
- Yu, X., Bao, B., Echigoya, Y., Yokota, T. Dystrophin-deficient large animal models: translational research and exon skipping. Am. J. Transl. Res. 7 (8), 1214-1231 (2015).
- Yin, H., Moulton, H., Betts, C., Wood, M. CPP-directed oligonucleotide exon skipping in animal models of Duchenne muscular dystrophy. Methods Mol Biol. 683, 321-338 (2011).
- Moulton, H. M., Moulton, J. D. Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim. Biophys. Acta. 1798 (12), 2296-2303 (2010).
- Morcos, P. A., Li, Y., Jiang, S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. BioTechniques. 45 (6), 613-614 (2008).
- Betts, C., et al. A New Generation of Peptide-oligonucleotide Conjugates With Improved Cardiac Exon Skipping Activity for DMD Treatment. Mol Ther Nucleic Acids. 14 (1), e38 (2012).
- Saito, T., et al. Antisense PMO found in dystrophic dog model was effective in cells from exon 7-deleted DMD patient. PLoS One. 5 (8), e12239 (2010).
- Yokota, T., et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 65 (6), 667-676 (2009).
- Melacini, P., et al. Cardiac and respiratory involvement in advanced stage Duchenne muscular dystrophy. Neuromuscul. Disord. 6 (5), 367-376 (1996).
- Guncay, A., Yokota, T. Antisense oligonucleotide drugs for Duchenne muscular dystrophy: how far have we come and what does the future hold. Future Med. Chem. 7 (13), 1631-1635 (2015).
- Fairbrother, W. G., Yeh, R. F., Sharp, P. A., Burge, C. B. Predictive identification of exonic splicing enhancers in human genes. Science. 297, 1007-1013 (2002).
- Cartegni, L., Wang, J., Zhu, Z., Zhang, M. Q., Krainer, A. R. ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res. 31, 3568-3571 (2003).
- Yokota, T., Hoffman, E., Takeda, S. Antisense oligo-mediated multiple exon skipping in a dog model of duchenne muscular dystrophy. Methods Mol Biol. 709, 299-312 (2011).
- Jenuth, J. P. The NCBI. Publicly available tools and resources on the Web. Methods Mol Biol. 132, 301-312 (2000).
- Yokota, T., et al. Extensive and Prolonged Restoration of Dystrophin Expression with Vivo-Morpholino-Mediated Multiple Exon Skipping in Dystrophic Dogs. Nucleic Acid Ther. , (2012).
- Summerton, J. E. Endo-Porter: a novel reagent for safe, effective delivery of substances into cells. Ann. N. Y. Acad. Sci. 1058, 62-75 (2005).
- Mangram, A. J., et al. Guideline for Prevention of Surgical Site Infection. Am J Infect Control. 27, 97-134 (1999).
- Reichman, D. E., Greenberg, J. A. Reducing Surgical Site Infections. A Review . Rev Obstet Gynecol. 2, 212-221 (2009).
- Mizuno, H., Nakamura, A., Aoki, Y., Ito, N., Kishi, S., Yamamoto, K., Sekiguchi, M., Takeda, S., Hashido, K. Identification of muscle-specific microRNAs in serum of muscular dystrophy animal models: promising novel blood-based markers for muscular dystrophy. PloS one. 6, (2011).
- Shimatsu, Y., et al. Major clinical and histopathological characteristics of canine X-linked muscular dystrophy inJapan, CXMDJ. Acta Myol. , 145-154 (2005).
- Evans, H., de Lahunta, A. Guide to the Dissection of the Dog. SAUNDERS. , (2009).
- Girish, V., Vijayalakshmi, A. Affordable image analysis using NIH Image/ImageJ. Indian J Cancer. 41 (47), (2004).
- Bearer, E. L., et al. Overview of image analysis, image importing, and image processing using freeware. Current protocols in molecular biology. 14, (2003).
- Shimatsu, Y., et al. Major clinical and histopathological characteristics of canine X-linked muscular dystrophy inJapan, CXMDJ. Acta Myol. 24, 145-1454 (2005).
- Beroud, C., et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum Mutat. 28, 196-202 (2007).
- Ferreiro, V., et al. Asymptomatic Becker muscular dystrophy in a family with a multiexon deletion. Muscle Nerve. 39, 239-243 (2009).
- Nakamura, A., et al. Follow-up of three patients with a large in-frame deletion of exons 45-55 in the Duchenne muscular dystrophy (DMD) gene. J Clin Neurosci. 15, 757-763 (2008).
- Yokota, T., Pistilli, E., Duddy, W., Nagaraju, K. Potential of oligonucleotide-mediated exon-skipping therapy for Duchenne muscular dystrophy. Expert Opin Biol Ther. 7, 831-842 (2007).
- Taniguchi-Ikeda, M., et al. Pathogenic exon-trapping by SVA retrotransposon and rescue in Fukuyama muscular dystrophy. Nature. 478, 127-131 (2011).
- Lee, J. J., Yokota, T. Antisense therapy in neurology. J Pers Med. 3, 144-176 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone