Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Shunt Surgery, Right Heart Catheterization, and Vascular Morphometry in a Rat Model for Flow-induced Pulmonary Arterial Hypertension
W tym Artykule
Podsumowanie
This protocol describes a surgical procedure to create a model for flow-induced pulmonary arterial hypertension (PAH) in rats and the procedures to analyze the principle hemodynamic and histological end-points in this model.
Streszczenie
In this protocol, PAH is induced by combining a 60 mg/kg monocrotalin (MCT) injection with increased pulmonary blood flow through an aorto-caval shunt (MCT+Flow). The shunt is created by inserting an 18-G needle from the abdominal aorta into the adjacent caval vein. Increased pulmonary flow has been demonstrated as an essential trigger for a severe form of PAH with distinct phases of disease progression, characterized by early medial hypertrophy followed by neointimal lesions and the progressive occlusion of the small pulmonary vessels. To measure the right heart and pulmonary hemodynamics in this model, right heart catheterization is performed by inserting a rigid cannula containing a flexible ball-tip catheter via the right jugular vein into the right ventricle. The catheter is then advanced into the main and the more distal pulmonary arteries. The histopathology of the pulmonary vasculature is assessed qualitatively, by scoring the pre- and intra-acinar vessels on the degree of muscularization and the presence of a neointima, and quantitatively, by measuring the wall thickness, the wall-lumen ratios, and the occlusion score.
Wprowadzenie
The goal of this method is to create a reproducible model for severe, flow-induced pulmonary arterial hypertension in rats and to measure its principle hemodynamic and histopathological end points.
Pulmonary arterial hypertension (PAH) is a clinical syndrome that encompasses a progressive increase in pulmonary vascular resistance leading to right ventricular failure and death. Within the superordinate disease spectrum of pulmonary hypertensive diseases (PH), PAH is the most severe form and one that remains without a cure1. The underlying arteriopathy in PAH is characterized by a typical form of vascular remodeling that occludes the vessel lumen. Muscularization of normal non-muscularized vessels and hypertrophy of the medial vessel layer are regarded as early disease phenomena in PAH, are also seen in other forms of PH2, and are thought to be reversible3. As PAH advances, the intimal layer begins to remodel, eventually forming characteristic neointimal lesions2. Neointimal-type pulmonary vascular remodeling is exclusive to PAH and is currently regarded to be irreversible4.
As PAH is a rare disease, advances in its pathobiological comprehension and development of novel therapies have relied heavily on animal models. The monocrotalin (MCT) model in rats is a simple single hit model that has been, and still is, used frequently. MCT is a toxin that causes injury to the pulmonary arterioles and regional inflammation5. 60 mg/kg MCT leads to an increase in the mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (PVR), and right ventricular hypertrophy (RVH) after 3 - 4 weeks6. The histomorphology is characterized by isolated medial hypertrophy without neointimal lesions5. The MCT rat model thus represents a moderate form of PH, and not PAH, although it is commonly presented as the latter.
In children with PAH associated with a congenital left-to-right shunt (PAH-CHD), increased pulmonary blood flow is regarded as the essential trigger for the development of neointimal lesions7,8,9. In rats, increased pulmonary blood flow can be induced by the creation of a shunt between the abdominal aorta and the vena cava, a technique first described in 199010. Alternatives to create increased pulmonary flow are by unilateral pneumonectomy or by subclavian to pulmonary artery anastomosis11. Conceptual disadvantages of these models consist of potential compensatory growth of the remaining lung and adaptive pathway activation induced by the pneumonectomy, or of iatrogenic injury of the pulmonary vasculature due to pulmonary artery anastomosis, both confounding the effects of increased pulmonary blood flow.
When an aorto-caval shunt is created and increased pulmonary blood flow is induced as a second hit in MCT-treated rats, characteristic neointimal lesions occur, and a severe form of PAH and associated right ventricular failure (RVF) develop 3 weeks after the increased flow12. The hemodynamic progression of PAH in this model can be assessed in vivo by echocardiography and right heart catheterization. The vascular histomorphology, vessel wall thickness, degree of arteriolar occlusion, and parameters for right ventricular failure form the pillars of the ex vivo characterization of PAH.
This method describes detailed protocols for the aorto-caval shunt (AC-shunt) surgery, right heart catheterization, and qualitative and quantitative assessment of vascular histomorphology.
Protokół
Procedures involving animal subjects have been approved by the Dutch Central Committee for Animal Experiments and the Animal Care Committee at University Medical Center Groningen (NL). Both Wistar and Lewis rats with weights between 180 and 300 g were used.
1. Housing and Acclimatization
- After arrival at the central animal facility, house rats in groups of 5 per cage. During a 7-day acclimatization period, accustom the rats to human handling, but do not perform any experimental procedures.
2. Preparation and Injection of Sterile Monocrotalin
- For 1 mL of 60 mg/mL monocrotalin (MCT) solution, weigh 60 mg of monocrotalin in a 2-mL tube. Add 700 µL of 0.9% NaCl. Add 200 µL of 1 M HCl. Warm the solution in the tube under hot running tap water and vortex it. Use 6 N NaOH to bring the pH towards 7.0. Use sterile technique for preparation of MCT for injection into rodents.
- Inject 1 mL of sterile 60 mg/mL MCT solution per kg subcutaneously in the neck (0.3 mL of 60 mg/mL MCT for a 300-g rat). NOTE: We prefer not to use smaller volumes due to the greater chance that the injected dose will not be appropriate.
3. Aorta-caval Shunt Surgery
- Anesthesia.
- Fill the induction chamber with 5% isoflurane/100% O2 (flow: 1 L/min) and place the rat in the chamber. Check for adequate depth of the anesthesia by performing a hind toe pinch. Weigh the rat.
- Shave and clean the abdomen over an area that is approximately 8 cm long and 3 cm wide. Place the rat on its back on a heat mat (37 °C) covered by a sterile mat.
- Place the snout in a ventilation mask/hood with 2 - 3% isoflurane/100% O2 (flow: 1 L/min). Check the depth of anesthesia by performing a hind toe pinch. Apply eye ointment to prevent dryness while under anesthesia.
- Shunt Surgery.
- Scrub the skin with chloride-hexidine for disinfection. Inject 0.01 mg/kg buprenorphine subcutaneously for post-operative analgesia.
- Use sterile instruments for surgery. Make an incision with a #10 scalpel blade in the abdomen on the midline, starting 1 cm below the diaphragm an extending down to just above the genitalia.
- Lift up the intestine with a cotton swab, cover the intestines in a sterile, wet gauze (0.9% NaCl), and place them to the left side of the animal.
- Use cotton swabs to separate the membranes that attach the abdominal aorta and the vena cava inferior to the surrounding tissues.
NOTE: Do not dissect the membranes between the aorta and the vena cava. - Using splinter forceps, remove the perivascular aortic fat just above the bifurcation, only on the right side of the aorta and only on the site where the needle will be inserted.
- Use cotton swabs to separate the aorta and vena cava from 2 mm superior to the site where the needle will be inserted in order to create space for a Biemer clamp.
- At this area, first place a loose ligature (5-0 suture) around the aorta. Create tension on the ligature by placing a Kocher clamp on it, and then place the Kocher superior to the incision (Figure 1A). Place the Biemer clamp just superior to the ligature (Figure 1A).
- Using a cotton swab, compress the vena cava as distally as possible to obstruct the flow (Figure 1A). Bend a needle (18 G in this protocol) into a 45-degree angle, with the orifice pointing towards the outside (Figure 1A).
- At an angle of 90 degrees, insert the needle in the aorta, just above the bifurcation, with the orifice of the needle pointing to the left (Figure 1A). Manipulate the tip of the needle to the left and insert it into the vena cava.
NOTE: The needle tip should now be visible in the vena cava (Figure 1B). - Use a second cotton swab to push the remaining blood in the aorta out of the insertion site to prevent thrombosis. Dry the area around the shunt with a sterile gauze in order for the glue to adequately stick.
- Pull the entire needle out of the aorta and immediately apply a drop of tissue glue onto the puncture site in the aorta. Make sure not to glue the cotton swab to the tissue. Unclamp the aorta.
- Verify the shunt manually by pulling on and releasing the ligature on the aorta proximal to the shunt. Loosening should color the vena cava distal to the shunt in bright red and create turbulence at the shunt site.
NOTE: Tightening will turn the blood in the vena cava back to dark red. - Place the intestines back in the animal. Close the muscle layer and skin with resorbable 4-0 sutures. Ventilate the animal with 100% O2 to recover from anesthesia.
NOTE: Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency.
- Sham Surgery.
- Perform all of the above procedures except for the insertion of the needle into the aorta.
- Post-surgical care.
- Place the rat in a single cage and into an incubator at 37 °C until the next morning.
- Around 6 h after surgery, inject 0.01 mg/kg buprenorphine subcutaneously for post-operative analgesia. Repeat the next morning if the rat shows signs of discomfort.
NOTE: The first 3 days after surgery, rats tend to eat and drink less (this is particularly important when chow or drinking water are mixed with drugs). Most rats show normal behavior 3 days after surgery. If not, monitor closely. Weight loss exceeding 15% in 1 week is considered abnormal, and such rats should be euthanized by the extraction of the circulating blood volume while under anesthesia.
4. Development of PAH
NOTE: In this protocol, the animal is euthanized by the extraction of the circulating blood volume while under anesthesia.
- Sacrifice 1 day after the surgery (MF8) for the early cellular and functional responses to increased pulmonary blood flow (e.g., gene up-regulation or early transcription factors).
- Sacrifice 1 week after the surgery (MF14) for an early-stage PAH vascular phenotype (medial hypertrophy without neointimal lesions).
- Sacrifice 2 weeks after the surgery (MF21) for an advanced-stage PAH vascular phenotype (marked medial hypertrophy and neointimal formation) with mild elevation in RVP and mPAP.
- Sacrifice 3 weeks after the surgery (MF28) for an end-stage PAH vascular phenotype (marked neointimal occlusion) and strong elevation in RVP and mPAP. Clinical signs of right ventricular failure are common in this stage.
- Sacrifice after day 28 (MF-RVF) for PAH-associated right ventricular failure (RVF), clinically defined as dyspnea, severe lethargy, and weight loss (< 10% in 1 week). Terminate rats when one of these signs is present. Frequently, rats develop these symptoms between days 28 and 35 and, if left unguarded, die spontaneously during this time interval.
5. Right Heart Catheterization
- Anesthesia.
- Fill the induction chamber with 5% isoflurane/100% O2 (flow: 1 L/min) and place the rat in the box. Check for adequate depth of the anesthesia by performing a hind toe pinch. Weigh the rat.
- Shave and clean the neck at the right-ventral side of the rat and, for the echocardiography protocol, the thorax and upper abdomen.
- Place the rat on its back on a heat mat (37 °C) and place the snout in a ventilation mask/hood with 2 - 3% isoflurane/100% O2 (flow: 1 L/min). The snout should be faced towards the researcher.
- Check depth of anesthesia. Be careful with rats with severe PH. If the heart rate decreases, reduce the depth of anesthesia. Preferable, perform all measurements within 20 min. Apply eye ointment to prevent dryness while under anesthesia.
- Echocardiography protocol.
- Perform the echocardiography according to the protocol described by Brittain et al. in JoVE13.
- Catheterization protocol.
NOTE: This protocol uses a rigid cannula with a preformed tip bent 20 degrees to guide the 15-cm silicon catheter with a ball 2 mm from the tip. A 20-G needle with its orifice slightly bent to the inside is used to insert the cannula into the right jugular vein (see the list of materials). Rats in any phase of PAH progression and control can be used in this protocol.- Disinfect the neck with chloride-hexidine. Make a 1.5-cm incision with a #10 scalpel blade in the right-ventral side of the neck, from the right collar bone to the mandibular bone.
- Spread the tissue using scissors. Using tweezers, gently pull the tissue apart until the jugular vein appears. Dissect the membranes around the jugular vein using splinter forceps.
- Put tension on the jugular vein by placing a loose ligature (5-0 suture) around the vessel. Increase the tension and tape the ligature onto the ventilation mask (Figure 2A).
- Downstream of the insertion site, place a loose ligature around the vessel to tighten after the cannula is in situ in order to prevent leakage and loss of pressure.
- Using the handles of a forceps, slightly bend the tip of a 20-G needle with the orifice to the inside to conduct the cannula with the catheter.
- Introduce the tip of the 20-G needle into the vein and quickly place the cannula containing the catheter inside the vessel. Pull out the needle, and then close the ligature that was prepared in step 5.3.4.
- Conduct the cannula containing the catheter into the jugular vein. The tip of the cannula is at a 20-degree curve (see step 5.3.5). Maneuver the cannula under the collar bone and advance a little to enter the right atrium (Figure 2C).
- To enter the right ventricle, point the tip of the cannula to the left, towards the heart (Figure 2D). On the bedside monitor, an RV pressure curve should appear, matching Figure 2D.
- When the RV pressure curve is constant, write down the systolic and diastolic right ventricular pressure 1 (sRVP1/dRVP1).
- Manipulate the tip of the cannula to the left and upwards. Advance the catheter within the cannula (Figure 2E).
- Advance the catheter into the main pulmonary artery (PA). No resistance should be felt when passing the pulmonary valve.
NOTE: When the catheter enters the main pulmonary artery, the diastolic pressure will rise. On the bedside monitor, a PA pressure curve should appear, matching Figure 2E. - When the PA pressure curve is constant, write down the systolic, diastolic, and mean PA pressure 1 (sPAP1, dPAP1, mPAP1).
- Further advance the catheter within the cannula until the ball at the tip of the catheter gets wedged in a pulmonary artery. Observe the pressure curve on the bedside monitor drop and match the wedge pressure curve in Figure 2F.
- When the wedge pressure curve is constant, write down the systolic, diastolic, and mean wedge pressure.
- Pull back the catheter slowly and subsequently measure and write down the values for sPAP2, dPAP2, mPAP2, sRVP2, and dRVP2, as displayed on the bedside monitor.
- When in the RV, slightly pull back the cannula and catheter to measure the mean right atrial pressure (RAP). The curve should match the RAP curve in Figure 2A.
NOTE: In this protocol, the rats are euthanized after the catheterization protocol by the extraction of the circulating blood volume while under anesthesia.
6. Morphology Assessment and Morphometry
NOTE: In this protocol, the animal is euthanized by the extraction of the circulating blood volume while under anesthesia. Rats in any phase of PAH progression and control can be used in this protocol.
- After sacrifice, take out the lungs by cutting the trachea about 5 mm above the bronchial bifurcation and the vessels that connect the lungs to the heart. Put the lungs in cold saline. Dissect the left lung. Cut the left main bronchus at the bifurcation.
- Fill a 50-mL syringe with 4% paraformaldehyde, attach a tube with a cannula to the syringe, and hang the syringe about a meter above the work table. Fit the cannula in the left main bronchus to passively fill the lung with paraformaldehyde. Handle paraformaldehyde with caution.
- Incubate the left lung in paraformaldehyde for 48 h.
- Dehydrate the left lung by incubating it consecutively in 70% ethanol (1 h), 80% ethanol (1 h), 90% ethanol (1 h), 100% ethanol (3 h), xylol (2 h), and paraffin (2 h).
- Embed the left lung in paraffin, with the hilum of the lung facing the cassette.
- Stain the paraffin-embedded, 4-µm lung sections using a Verhoeff or Elastica-van Gieson staining, as per manufacturer's instructions29. Make sure the elastic laminae are well differentiated (as in Figure 3). Scan the stained sections at 40X magnification.
- Divide the lung into 4 quadrants. In each quadrant, find 10 vessels with an outer diameter < 50 µm (intra-acinar) and 10 vessels with an outer diameter > 50 µm (pre-acinar). Take a picture (2 x 40 pictures per lung). Zoom in randomly up to 20x magnification and photograph every vessel in this field of view to minimize selection bias.
- Exclude vessels that have a longest/shortest diameter ratio of > 2, an incomplete circular shape, or a collapse of more than one quarter of the vessel wall.
NOTE: An example of an excluded vessel is shown in Figure 3b Make each picture on the same magnification (40X) and include a scale bar. - Open ImageJ and the first picture. Draw a straight line on the scale bar in the picture to set the scale via "Analyse" and "Set scale." For "known distance," use the value on the picture's scale bar. Use micrometers (µm) as the unit of length. Set the scale to global.
- Using "freehand selections," draw a line on the inner border of the luminal area (Figure 3), and use "measure" (crtl m) to measure this area. Then, draw a line around the outer elastic lamina (Figure 3) to measure the total vessel area.
- Calculate the luminal and outer diameter (
 ) using
) using  .
. - Calculate wall thickness using
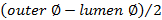 .
. - Calculate the wall/lumen ratio using
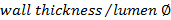 .
. - Calculate the occlusion score using
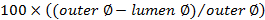 .
. - Score the vessel on muscularization (no, partial, or total muscularization) (Figure 3B).
NOTE: Vessels with a double elastic lamina for more than half the circumference are defined as totally muscularized. Vessels with a double elastic lamina less than half the circumference are defined as partially muscularized. - Score the vessel on the presence of a neointima (yes or no) (Figure 3C).
NOTE: Vessels without a clearly-defined internal elastic lamina combined with (often eccentric) luminal occlusion are defined as neointimal lesions.
Wyniki
Representative results are presented in Figure 4. The presented results show characteristics of MCT+FLOW in Lewis rats in the following groups: Control (n = 3), MF8 (n = 5), MF14 (n = 5), MF28 (n = 5), and MF-RVF (n = 10). Statistical analyses were performed using the one-way ANOVA with Bonferroni correction.
60 mg/kg MCT and increased pulmonary blood flow lead to a mean rise in systolic right ventricular pressu...
Dyskusje
This method describes the surgical procedure of an aorto-caval shunt in rats pre-treated with MCT to create flow-induced PAH and the techniques to assess the principle hemodynamic and histopathological end points that characterize PAH and this model.
Critical Steps within the Protocol and Troubleshooting
Surgery and post-surgery. During the aorto-caval shunt surgery, the most critical step is the dissection of the aorta and vena cava. The membranes that enclose the aor...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This study was supported by the Netherlands Cardiovascular Research Initiative, the Dutch Heart Foundation, the Dutch Federation of University Medical Centers, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences (CVON nr. 2012-08, PHAEDRA, The Sebald fund, Stichting Hartekind).
Materiały
| Name | Company | Catalog Number | Comments |
| Shunt Surgery | |||
| Sterile surgical gloves | |||
| Duratears Eye ointment | Alcon | 10380 | |
| Chloride-Hexidine | |||
| Cotton swabs | |||
| Histoacryllic tissue glue | B. Braun Medical | 1050052 | |
| Silkam 5-0 sutures black non-resorbable | B. Braun Medical | F1134027 | |
| Safil 4-0 sutures violet resorbable | B. Braun Medical | ||
| 18 G needle | Luer | NN1838R BD | tip bent in 45 degrees orifice to the outside |
| Gauzes 10 x 10 cm | Paul Hartmann | 407825 | |
| Temgesic Buprenorphine | RB Pharmaceuticals | 5429 | subcutaneous injection |
| Sodium Chloride 0.9% | |||
| Ventilation mask Rat | |||
| Scalple blade | |||
| Biemer clamp 18 mm, 5 mm opening | AgnTho | 64-562 | |
| Heat mat | |||
| Kocher Clamp | |||
| Shaving machine | |||
| Microscope | Leica | ||
| Right Heart Catheterization | |||
| Sterile surgical gloves | |||
| Eye ointment | Duratears | ||
| Chloride-Hexidine | |||
| Cotton swabs | |||
| Gauzes 10 x 10 cm | Paul Hartmann | 407825 | |
| Silkam 5-0 sutures black non-resorbable | B. Braun Medical | F1134027 | |
| Needle 20 G | Luer | Tip slightly bent to the inside | |
| Cannula 20 G | Luer | to introduce catheter, tip pre-formed in 20 degrees | |
| Silastic Catheter 15 cm long | 0.5 mm ball 2 mm from tip | ||
| Pressure transducer | Ailtech | ||
| Bedside monitor Cardiocap/5 | Datex-Ohmeda | ||
| Shaving machine | |||
| 10 mL Syringe | |||
| Sodium Chloride 0.9% | for flushing | ||
| Vascular Morphology | |||
| 50 mL Syringe | |||
| 4% Formaldehyde | |||
| 18 G cannula with tube | |||
| Verhoef staining kit | Sigma-Aldrich | HT254 | http://www.sigmaaldrich.com/catalog/product/sigma/ht254?lang=en®ion=US |
| Digital slide scanner | Hamamatsu | C9600 | |
| ImageJ | |||
| Elastic (Connective Tissue Stain) | Abcam | ab150667 | http://www.abcam.com/elastic-connective-tissue-stain-ab150667.html http://www.abcam.com/ps/products/150/ab150667/documents/ab150667-Elastic%20Stain%20Kit%20(website).pdf |
Odniesienia
- Hoeper, M. M., Bogaard, H. J., Condliffe, R., et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 62, D42-D50 (2013).
- Stacher, E., Graham, B. B., Hunt, J. M., et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 186 (3), 261-272 (2012).
- Levy, M., Maurey, C., Celermajer, D. S., et al. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol. 49 (7), 803-810 (2007).
- Sakao, S., Tatsumi, K., Voelkel, N. F. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 43 (6), 629-634 (2010).
- Gomez-Arroyo, J. G., Farkas, L., Alhussaini, A. A., et al. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 302 (4), L363-L369 (2012).
- Jones, J. E. Serial noninvasive assessment of progressive pulmonary hypertension in a rat model. Am J Physiol - Heart Circ Physiol. 283 (1), 364-371 (2002).
- Hoffman, J. I., Rudolph, A. M., Heymann, M. A. Pulmonary vascular disease with congenital heart lesions: Pathologic features and causes. Circulation. 64 (5), 873-877 (1981).
- van Albada, M. E., Berger, R. M. Pulmonary arterial hypertension in congenital cardiac disease--the need for refinement of the evian-venice classification. Cardiol Young. 18 (1), 10-17 (2008).
- Dickinson, M. G., Bartelds, B., Borgdorff, M. A., Berger, R. M. The role of disturbed blood flow in the development of pulmonary arterial hypertension: Lessons from preclinical animal models. Am J Physiol Lung Cell Mol Physiol. 305 (1), L1-L14 (2013).
- Garcia, R., Diebold, S. Simple, rapid, and effective method of producing aortocaval shunts in the rat. Cardiovasc Res. 24 (5), 430-432 (1990).
- Okada, K., Tanaka, Y., Bernstein, M., Zhang, W., Patterson, G. A., Botney, M. D. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol. 151 (4), 1019-1025 (1997).
- van Albada, M. E., Schoemaker, R. G., Kemna, M. S., Cromme-Dijkhuis, A. H., van Veghel, R., Berger, R. M. The role of increased pulmonary blood flow in pulmonary arterial hypertension. Eur Respir J. 26 (3), 487-493 (2005).
- Brittain, E. Echocardiographic assessment of the right heart in mice. JVis Exp. (e81), (2013).
- Dickinson, M. G., Bartelds, B., Molema, G., et al. Egr-1 expression during neointimal development in flow-associated pulmonary hypertension. Am J Pathol. 179 (5), 2199-2209 (2011).
- Borgdorff, M. A., Bartelds, B., Dickinson, M. G., Steendijk, P., de Vroomen, M., Berger, R. M. Distinct loading conditions reveal various patterns of right ventricular adaptation. Am J Physiol Heart Circ Physiol. 305 (3), H354-H364 (2013).
- Ruiter, G., de Man, F. S., Schalij, I., et al. Reversibility of the monocrotaline pulmonary hypertension rat model. Eur Respir J. 42 (2), 553-556 (2013).
- van Albada, M. E., Bartelds, B., Wijnberg, H., et al. Gene expression profile in flow-associated pulmonary arterial hypertension with neointimal lesions. Am J Physiol Lung Cell Mol Physiol. 298 (4), L483-L491 (2010).
- Dickinson, M. G., Kowalski, P. S., Bartelds, B., et al. A critical role for egr-1 during vascular remodelling in pulmonary arterial hypertension. Cardiovasc Res. 103 (4), 573-584 (2014).
- van der Feen, D. E., Dickinson, M. G., Bartelds, M. G., et al. Egr-1 identifies neointimal remodeling and relates to progression in human pulmonary arterial hypertension. Jheart lung transplant. 35 (4), 481-490 (2016).
- Rungatscher, A. Chronic overcirculation-induced pulmonary arterial hypertension in aorto-caval shunt. Microvasc Res. 94, 73-79 (2014).
- O'Blenes, S. B., Fischer, S., McIntyre, B., Keshavjee, S., Rabinovitch, M. Hemodynamic unloading leads to regression of pulmonary vascular disease in rats. J Thorac Cardiovasc Surg. 121 (2), 279-289 (2001).
- Sakao, S., Taraseviciene-Stewart, L., Lee, J. D., Wood, K., Cool, C. D., Voelkel, N. F. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 19 (9), 1178-1180 (2005).
- Spiekerkoetter, E. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 123 (8), 3600-3613 (2013).
- Nickel, N. P., Spiekerkoetter, E., Gu, M., et al. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med. 191 (11), 1273-1286 (2015).
- Meloche, J., Potus, F., Vaillancourt, M., et al. Bromodomain-containing protein 4: The epigenetic origin of pulmonary arterial hypertension. Circ Res. 117 (6), 525-535 (2015).
- Happé, C. M. Pneumonectomy combined with SU5416 induces severe pulmonary hypertension in rats. Am J Physiol Lung Cell Mol Physiol. 310 (11), L1088-L1097 (2016).
- Ranchoux, B., Antigny, F., Rucker-Martin, C., et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 131 (11), 1006-1018 (2015).
- de Raaf, M. A. SuHx rat model: Partly reversible pulmonary hypertension and progressive intima obstruction. Eur Respy J. 44 (1), 160-168 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone