Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Determination of the Relative Potency of an Anti-TNF Monoclonal Antibody (mAb) by Neutralizing TNF Using an In Vitro Bioanalytical Method
W tym Artykule
Podsumowanie
A protocol for the determination of the relative anti-apoptotic activity of an anti-TNFα mAb using a neutralization mechanism with WEHI 164 cells is presented here. This protocol is useful for comparing the neutralization strength of different molecules with the same biological functionality.
Streszczenie
This protocol shows the measurement of the apoptotic activity neutralization of TNFα in a mouse fibroblast cell model (WEHI 164) using an anti-TNFα mAb. In addition, this protocol can be used to evaluate other anti-TNFα molecules, such as fusion proteins. The cellular model employed here is sensitive to TNFα-mediated apoptosis when an additional stress factor is induced in cell culture conditions (e.g., serum deprivation). This procedure exemplifies how to execute this analytical assay, highlighting the key operations relating to the sample preparation, cell dilution, apoptosis induction, and spectrophotometric measurements that are critical to ensure successful results. This protocol reveals the best-performance conditions relating to apoptosis induction and efficient signal recording, leading to low uncertainty values.
Wprowadzenie
Biological potency is the quantitative measure of biological activity based on the assayed product attributes that are linked to the relevant biological properties, whereas quantity (expressed in mass) is a physicochemical measure of protein content. Potency tests, along with other analytical methodologies, are performed as part of product conformance, stability, and comparability studies. In this sense, potency measurements are used to demonstrate that product batches meet the critical quality attributes (CQAs) or acceptance criteria during all phases of clinical trials and after market approval.
Apoptosis is programmed cell death, naturally occurring when cells are infected with a virus or when the cells are stressed by an environmental factor that compromises cellular viability and function1,2. Among others, apoptosis inhibition, or biological neutralization, is one of the principally known therapeutic mechanisms of mAbs, particularly in the treatment of chronic diseases, such as immune-mediated inflammatory disorders. Anti-TNFα molecules exert their therapeutic properties by blocking the interaction of tumor necrosis factor alpha (TNFα) with the p55 and p75 cell surface receptors3, thus preventing signal pathways that finally lead to cellular apoptosis.
TNFα can produce inflammation in some chronic illnesses4. TNFα is spuriously secreted into the extracellular milieu by macrophages, which are sentries of the innate immune system and the main actors in this kind of disease5. As a common path, TNFα deregulation is associated with the pathogenesis of these illnesses.Without control and under constant induction and cell stress, TNFα induces cell death and tissue degeneration, ultimately leading to rheumatoid arthritis, Crohn's disease, and other pathological profiles6.
TNF antagonists that block the interaction between TNF and its receptors have been increasingly used as an effective therapy to reduce symptomatology and hinder the progression of these diseases. Nowadays, anti-TNFα drug products are widely used to control the systemic concentration of this cytokine, thus preventing further degeneration of involved tissues. In this sense, providing a reproducible and robust bioassay to describe the specific ability of a drug to achieve its biological effect is imperative.
In this protocol, critical steps-identified during the development of a neutralization assay-for the successful measurement of biological potency are highlighted, with a particular emphasis on the skills needed to execute the bio-analytical method. This bio-analytical method provides useful comparability information between different batches or anti-TNFα drug products when compared to a clinically tested reference substance.
Protokół
1. Preparation of the Media and Solutions
- Prepare the culture medium: RPMI-1640 with 10% FBS, pH 7.4.
- Prepare assay culture medium: RPMI-1640 without phenol red but with 1% FBS, pH 7.4.
- Prepare cell wash solution: DPBS Mg- and Ca-free solution with 0.02% EDTA, pH 7.4.
- Prepare cell detachment solution: 0.125% trypsin with 1 mM EDTA.
- Thaw 100 mL of a 0.25% solution of trypsin-EDTA and transfer to a sterile 500-mL flask.
- Mix with 100 mL of cell wash solution and dispense 15 mL aliquots into 15 mL sterile tubes. Store at -70 to -80 °C until use.
- Filter these solutions through a 0.22-µm membrane and warm up to 37 °C for at least 30 min prior to use.
- Prepare apoptosis-induction stock solution TNFα solution at 3.3 µg/mL.
- Dissolve 20 µg of TNFα with 500 µL of filter-sterilized water in its primary container and mix until complete dissolution.
- Transfer into a 15 mL sterile tube and add 5.5 mL of DPBS Mg- and Ca-free solution to this tube. Mix gently using a vortex mixer.
- Aliquot the solution into 70 µL portions. Dispense each aliquot into 0.5 mL microtubes and store at -80 °C.
- Prepare apoptosis induction solution : TNFα solution at 40 ng/mL.
- Thaw an aliquot of the apoptosis induction stock solution, incubating it in a water bath at 25 ˚C for 10 min.
- Dilute the apoptosis induction stock solution to 40 ng/mL by adding 61 µL of 3.3 µg/mL TNFα solution to 4.939 mL of assay culture medium in a 15 mL sterile tube.
- Mix by vortex mixer for 10 s; this solution must be prepared freshly before use.
- Warm up the solution to 37 °C for at least 30 min prior to use in th eneutralization assay.
- Prepare the substrate solution: caspase 3/7 Glo solution7,8.
- Thaw the caspase buffer solution (caspase 3/7 Glo buffer) 12 h before use.
- Let the caspase buffer solution and the substrate (caspase 3/7 Glo substrate) sit separately at 25 ± 5 °C for 30 min prior to mixing.
- Transfer 10 mL of the caspase buffer solution to the substrate vial and mix by inversion.
- Keep at 25 ± 5 °C, light-protected until use.
NOTE: The solution is stable for 6 h at room temperature.
2. Cell Culturing and Counting
- Cell thawing and the first subculture.
- Remove one vial with WEHI 164 cells9 from a freezer at -80 ˚C and transfer them to an ice-bath.
- Pipette up and down with 1 mL of pre-warmed culture medium until the frozen cells completely thaw.
- Dispense 9 mL of pre-warmed culture medium onto a 15 mL sterile tube.
- Transfer the cell suspension into the 15-mL sterile tube and mix gently five times by inversion.
- Centrifuge the cell suspension at 125 x g for 3 min. Discard the supernatant and disaggregate the cell pellet.
- Add 5 mL of culture medium to the tube . Mix until the cells are completely resuspended.
- For cell counting, transfer 50 µL of the cell suspension to a 500 µL microtube and mix with 50 µL of 0.4% trypan blue. Count the cells and adjust to 0.5 x 106 cells/mL. See step 2.2, below.
- Add 13 mL of pre-warmed culture medium to a 75 mL cell culture flask.
- Dispense enough cell suspension volume from step 2.1.6 to achieve 0.5 x 106 cells/mL in the cell culture flask and incubate at 37 °C and 5% CO2 overnight.
- Cell counting.
NOTE: See reference10.- Using the solution from step 2.1.6, transfer 0.05 mL to a hemocytometer and determine the cell density under a microscope using trypan blue exclusion.
- Quantify the total number of cells and viable cells.
- Adjust the cell suspensions to 0.5 x 106 cells/mL.
Equation 1
Vculture medium(mL) =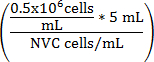
Vculture medium(mL) = (5mL - Vcell suspension)
Vculture medium(mL) = Adjusted volume of WEHI 164 cell suspension
NVC = Number of viable WEHI 164 cells/mL
Vculture medium(mL) = Assay culture medium volume added to the cell suspension to achieve 0.5 x 106 cells/mL
0.5 x 106 = Target cell density
- Cell detachment and the second and third subculture.
NOTE: A vacuum system can be used to remove the solutions from the flasks. Disposable or glass sterile pipettes can be used. If the pipette has a cotton clog at the top, it must be removed before use.- Remove the culture medium from the cell culture T-flask using a 1 mL sterile pipette and a vacuum.
- Dispense 5 mL of cell wash solution into the culture T-flask, mix gently, and discard the solution. Repeat this step twice.
NOTE: The complete removal of the culture medium is critical for efficient cell detachment. - Add 15 mL of cell detachment solution to the T-flask and let stand for 3 min in an incubator at 37 °C and 5% CO2.
- Verify the absence of attached cells in the flask inner wall under the microscope. Remove the cells from the culture T-flask using a 20 mL sterile pipette and dispense them into a 50-mL sterile tube.
- Centrifuge the cell suspension at 125 x g for 3 min. Discard the supernatant and resuspend the pellet with another 5 mL of culture medium.
- Count the cells and add enough culture medium to reach the desired cell concentration according to Equation 1.
- Add this suspension to a 72 cm2 T-flask and incubate overnight at 37 °C and 5% CO2.
- Subculture the cells at least two times before using them in the neutralization assay. Repeat steps 2.3.1-2.3.8 for the next two days.
- Assay cell suspension.
- Select a WEHI 164 subculture that has at least three passes. See step 2.1.
- Detach and count the cells according to steps 2.2 and 2.3 of this protocol.
- Dilute the cell suspension according to Equation 1 to 0.5 x 106 cells/mL.
- Use this cellular suspension for the neutralization assay. Mix all cell suspensions by vortex mixer prior to use.
3. Antibody Preparation and Dilutions
- Quantitation of the mAbs.
- Determine the concentration of reference substance, control sample, and analytical sample through UV absorption at 280 nm using their mass extinction coefficient (1.39)11.
NOTE: Original concentrations could be taken from drug product labels. However, this must be verified by UV absorption.
- Determine the concentration of reference substance, control sample, and analytical sample through UV absorption at 280 nm using their mass extinction coefficient (1.39)11.
- mAb dilutions.
- Dilute all samples independently in triplicate, with DPBS Mg- and Ca-free solution in 2 mL microtubes, down to 2 mg/mL. Confirm this concentration by UV absorption in triplicate, using DPBS Mg- and Ca-free solution as the blank.
- Mix the stock protein solutions for 5 s using a vortex mixer.
- Dilute 100 µL of each 2 mg/mL mAb solution with 0.9 mL of the assay culture medium.
- Mix for 5 s by vortex mixer.
NOTE: These solutions have a concentration of 200 µg/mL. Dilutions must be done for each triplicate. - Dilute 10 µL of each 200 µg/mL mAb solution with 0.99 mL of the assay culture medium. Mix for 5 s using a vortex mixer. These solutions have a concentration of 2 µg/mL. Perform serial dilutions for each triplicate before using them in the neutralization assay.
- Make anti-TNFα mAb dilutions in three independent microplates. Make a duplicate from each independent triplicate and dispense them in one microplate, as indicated in Table 1. Reference Substance
Table 1: Microplate sample arrays. A complete neutralization assay must be run in three microplates within coordinates B2 to G11. Random dispensing of reference, analytical, and control samples allow researchers to verify any bias in the assay.Plate 1 Plate 2 Plate 3 Wells Sample Wells Sample Wells Sample B2:B11 Reference Substance B2:B11 Control Sample B2:B11 Analytical Sample C2:C11 C2:C11 C2:C11 D2:D11 Analytical Sample D2:D11 Reference Substance D2:D11 Control Sample E2:E11 E2:E11 E2:E11 F2:F11 Control Sample F2:F11 Analytical Sample F2:F11 Reference Substance G2:G11 G2:G11 G2:G11 - Perform mAb dilutions of each reference, sample, or control, as shown in Table 2.
NOTE: The anti-TNFα mAb concentrations described in this table are not the final concentrations in the neutralization assay.
Table 2: Anti-TNFα mAb dilutions. Serial dilutions of anti-TNFα mAbs are demonstrated in this table. Final concentrations described in this table are not the concentrations in the assay, where anti-TNFα mAbs were diluted by a factor of 3 (mAb dilution + culture medium + cells suspension). Lines in bold represent dilutions coming from lines 3, 5, 7, 9, and 10; non-bolded lines represent dilution from lines 3, 4, and 6. These serial dilutions are done just before performing the neutralization assay. Care must be taken to mix by pipetting up and down three times before dispensing the dilutions.Plate Column Volume of Assay culture medium (μL) Volume of Reference Substance, Analytical Sample or Control Sample (uL) Concentration in the Assay Plate (ng/mL) 2 0 230 2000 3 150 150 from line 2 1000 4 75 75 from line 3 500 5 100 50 from line 3 333 6 75 75 from line 4 250 7 75 75 from line 5 166 8 75 75 from line 6 125 9 75 75 from line 7 83 10 75 75 from line 9 41 11 150 75 from line 10 13 - Keep the plates at 25 ± 5 °C until use.
4. Neutralization Assay with WEHI 164 Cells
- Mix by vortexing all cell suspensions (0.5 x 106 cells/mL) prior to dispensing at any step of this protocol.
NOTE: In this section, warm each solution to 37 °C for 30 min prior to use. - Transfer 50 µL of the cell suspension to each of the 60 wells of the microplates, moving from column 2 to 11 and line B to G.
- Transfer 50 µL of mAb reference, sample, and control dilutions into microplates. Follow the pattern depicted in Figure 1.
- Add 50 µL of the apoptosis induction solution to each well.
- Use cellular controls of 50 µL of WEHI 164 cells, dispensed in three wells. Bring each well to a final volume of 150 µL with assay culture medium.
- Use a cytotoxicity control of a mixture of 50 µL of WEHI 164 cells plus 50 µL of apoptosis induction solution. Bring each well to a final volume of 150 µL with assay culture medium.
- For the TNFα control, use 50 µL of the apoptosis induction solution and bring it to 150 µL with the assay culture medium.
- For the blank, use 150 µL of the assay culture medium alone.
- Fill the remaining wells with 150 µL of culture medium to avoid plate evaporation effects.
- Repeat steps 4.1.1-4.1.9 twice in two other microplates.
NOTE: The mAb final concentrations in the microplate are:0.666, 0.333, 0.167, 0.111, 0.083, 0.056, 0.042, 0.028, 0.014, and 0.004 µg/mL. - Load the samples in microplates, as indicated in Figure 1.
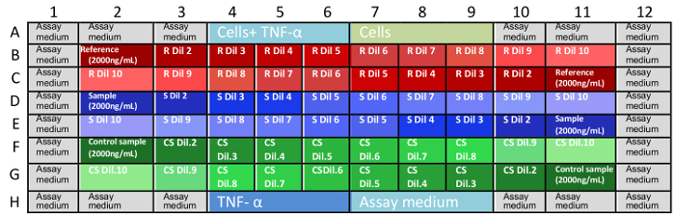
Figure 1: Disposition of samples in the assay plates. B1 to G11 are well coordinates in the microplates and describe the positions where the sample dilutions are placed. Missing coordinates are wells filled with controls and assay culture medium (A1-A12 and H1-H12). This random distribution of samples (forward and reverse dilutions in the microplates) helps to eliminate bias in the results due to the evaporation of medium or other variables. It is best that each microplate is done by one analyst at a time. R: Reference, S: sample, CS: control sample, Dil: dilution. Please click here to view a larger version of this figure. - Incubate the three plates at 37 °C and 5% CO2 for 16 ± 2 h.
- Let the caspase 3/7 Glo reagent stand at 25 ± 5 °C for 30 min before use.
- Add 100 µL of this reagent to all wells, including the samples and controls.
- Shake the plates using a microplate vortex mixer for 3 min at 25 ± 5 °C immediately after dispensing into the wells.
- Incubate the plates for 2.5 ± 0.5 h at 25 ± 5 °C, protected from light.
- Insert the microplates into the luminometer and complete the next section.
5. Analysis of Results
- Using a software for luminescence detection, select the luminescence mode and endpoint function.
- Select a 96-well clear-bottom plate and its 80 internal wells, excluding columns 1 and 12.
- Select an integration time of 1,250 ms and 10 s for mixing the microplate before reading.
- Select the wells where reference substance, analytical substance, and control sample will be placed and identify with their corresponding concentrations.
- Read the samples placed in the microplates with the luminometer.
- Use a fourth parameter equation for the analyses of results. Graph a dose-response curve, as depicted in Figure 2.
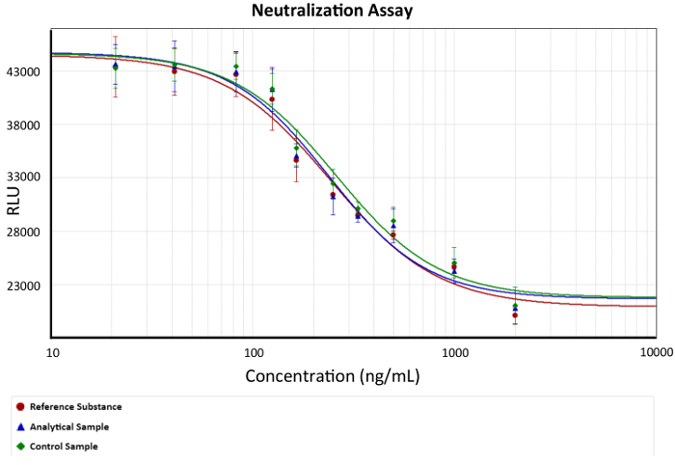
Figure 2: Dose-response curve. Anti-TNFα mAb concentration versus luminescence (cell viability) is depicted. A fourth parameter equation describing the anti-TNFα protection of mAbs was used as a model. EC50 is the concentration of mAb that can neutralize the amount of TNFα that cause 50% cell death in each assay, exemplified in the graph as the change in slope. Bars describe the standard deviation of luminescence for each mAb concentration. x represents anti-TNFα Ab concentration and is depicted as a logarithmic function in ng/mL, while y represents the luminescence response in arbitrary luminescence units. Please click here to view a larger version of this figure.
NOTE: In the fourth parameter equation, C is the effective concentration 50 (EC50). This value will be used to compare the reference substance, analytical sample, and control sample by means of the effector function. - For calculating the relative potencies, fix the reference substance to 100% and calculate the potencies of the sample and control accordingly.
NOTE: Those values are depicted in Figure 3.
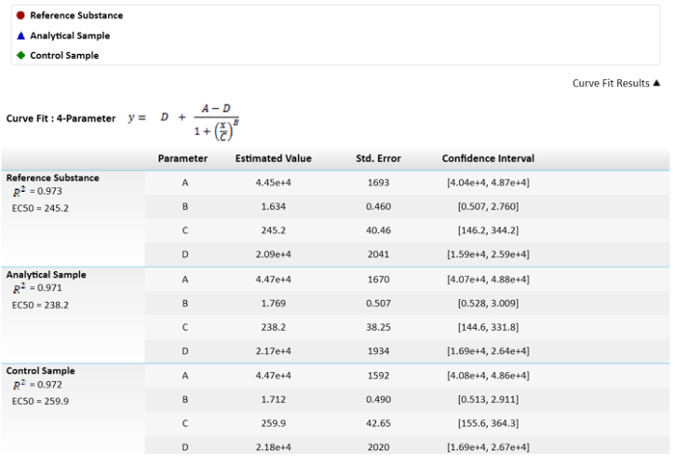
Figure 3: Mathematical equation used for calculating the EC50s and their values. EC50 values, or C parameters, have their uncertainty depicted as standard error. A comparison of EC50s between the sample and reference results of relative potency is also depicted. The confidence interval is calculated with an α = 0.05. Please click here to view a larger version of this figure.
Wyniki
Dose-response Graph (with Controls)
Figure 1 represents the luminescence response versus mAb concentration. This sigmoidal function exemplifies caspase 3 and 7 release in the assay culture medium due to cell lysis. Cell death is enhanced by serum starvation plus TNFα signaling induction. Therefore, the anti-TNFα molecule (mAb) interacts with the cytokine, inhibiting (by steric hindrance) its interaction with the TNF cell recep...
Dyskusje
This characterization helps to determine a priori the biological behavior of a molecule under development before expensive and time-consuming clinical trials are conducted. It is also useful for the batch-to-batch release of an approved drug product. It is worth mentioning that these assays are useful for determining if a molecule has an adequate biological effect regarding its mechanism of action. The bio-analytical method presented in this tutorial is critically important to the comparison of different anti-TN...
Ujawnienia
The authors have nothing to disclose
Podziękowania
This work was supported by the National Council of Science and Technology (CONACYT), Mexico grant PEI CONACYT 2015 220333, without participation in the design of the study.
Materiały
| Name | Company | Catalog Number | Comments |
| WEHI 164 | ATCC | CRL-1751 | Fibrosarcoma cells from Mus musculus |
| RPMI-1640 Medium | ATCC | 30-2001 | Store medium at 2 °C to 8 °C |
| RPMI 1640 Medium, no phenol red | GIBCO | 11835-030 | Store medium at 2 °C to 8 °C |
| Trypsin-EDTA(0.25%),phenol red | GIBCO | 25200-056 | Store medium at -10 °C to -20 °C |
| DPBS, no calcium, no magnesium | GIBCO | 14190-136 | Store medium at 2 °C to 8 °C |
| Recombinant Human TNF-alpha Protein | R&D Systems | 210-TA-020 | Store at -20 °C to -70 °C |
| Fetal Bovine Serum (U.S), Super Low IgG | HyClone | SH3089803 | Store at -10 °C to -20 °C |
| Fetal Bovine Serum (U.S.), Characterized | HyClone | SH3007103 | Store at -10 °C to -20 °C |
| Caspase-Glo 3/7 Assay kit | Promega | G8093 | Store the Caspase-Glo. 3/7 Substrate and Caspase-Glo. 3/7 Buffer at –20 ºC protected fromLight |
| EDTA, Disodium Salt, Dihydrate, Crystal, A.C.S. Reagent | J.T.Baker | 8993-01 | -- |
| Sample mAb Adalimumab | Probiomed | NA | Final concentrations in the microplate are: 0.666, 0.333, 0.167, 0.111, 0.083, 0.056, 0.042, 0.028, 0.014 and 0.004 μg/mL |
| Reference and Control mAb Adalimumab | Abbvie | NA | Final concentrations in the microplate are: 0.666, 0.333, 0.167, 0.111, 0.083, 0.056, 0.042, 0.028, 0.014 and 0.004 μg/mL |
| Microplate Reader | Molecular Devices | 89429-536 | SpectraMax M3 Multi-Mode |
| Microplate reader Software | Molecular Devices | -- | SoftMax Pro 6.3 GxP |
| Incubator | Revco | 30482 | Revco RNW3000TABB Forced-Air CO2 |
| Laminar Flow Hood | The Baker Company | 200256 | Baker SG603A-HE | High Efficiency, Class II Type A2 |
Odniesienia
- Elmore, S. Apoptosis: A Review of programmed cell death. Toxicol Patho. 35 (4), 495-516 (2007).
- Darwish, R. S. Regulatory mechanisms of apoptosis in regularly dividing cells. Cell Health Cytoskelet. 2 (1), 59-68 (2010).
- Tracey, D., et al. Tumor necrosis factor antagonist mechanism of action: A comprehensive review. Pharmacol Ther. 117 (2), 244-279 (2008).
- Körner, H., Sedgwick, J. Tumour necrosis factor and lymphotoxin: Molecular aspects and role in tissue-specific autoimmunity. Immunol Cell Biol. 74 (5), 465-472 (1996).
- Wong, M., et al. TNFa blockade in human diseases: Mechanisms and future directions. Clin Immunol. 126 (2), 121-136 (2008).
- Furst, D. E., Wallis, R., Broder, M., Beenhouwer, D. O. Tumor necrosis factor antagonists: different kinetics and/or mechanisms of action may explain differences in the risk for developing granulomatous infection. Semin Arthritis Rheum. 36 (3), 159-167 (2006).
- Karvinen, J., et al. Homogeneous time-resolved fluorescence quenching assay (LANCE) for caspase-3. J Biomol Screen. 7 (3), 223-231 (2002).
- Ren, Y. G., et al. Differential regulation of the TRAIL death receptors DR4 and DR5 by the signal recognition particle. Mol Biol Cell. 15 (11), 5064-5074 (2004).
- Sud, D., Bigbee, C., Flynn, J. L., Kirschner, D. E. Contribution of CD8+ T cells to control of Mycobacterium tuberculosis infection. J Immunol. 176 (7), 4296-4314 (2006).
- Strober, W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. Apendix 3, 3 (2001).
- Ramasubramanyan, N., et al. . Low acidic species compositions and methods for producing and using the same. 1, (2014).
- Masters, J. R., Stacey, G. N. Changing medium and passaging cell lines. Nat Protoc. 2 (9), 2276-2284 (2007).
- Eskandari, M. K., Nguyen, D. T., Kunkel, S. L., Remick, D. G. WEHI 164 subclone 13 assay for TNF: sensitivity, specificity, and reliability. Immunol Invest. 19 (1), 69-79 (1990).
- Hora, M. S., Rana, R. K., Smith, F. W. Lyophilized formulations of recombinant tumor necrosis factor. Pharm Res. 9 (1), 33-36 (1992).
- Ponnappan, S., Ponnappan, U. Aging and immune function: molecular mechanisms to interventions. Antiox Redox Signal. 14 (8), 1551-1585 (2011).
- Matsumaru, K., Ji, C., Kaplowitz, N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. 37 (6), 1425-1434 (2003).
- Camacho-Villegas, T., Mata-Gonzalez, T., Paniagua-Solis, J., Sanchez, E., Licea, A. Human TNF cytokine neutralization with a vNAR from Heterodontus francisci shark: a potential therapeutic use. mAbs. 5 (1), 80-85 (2013).
- Männel, D. N., Falk, W. Optimal induction of tumor necrosis factor production in human monocytes requires complete S-form lipopolysaccharide. Infect Immun. 57 (7), 1953-1958 (1989).
- Lis, K., Kuzawińska, O., Bałkowiec-Iskra, E. Tumor necrosis factor inhibitors-state of knowledge. Arch Med Sci. 10 (6), 1175-1185 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone