Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Whole-cell Patch-clamp Recordings of Isolated Primary Epithelial Cells from the Epididymis
W tym Artykule
Podsumowanie
We present a protocol that combines cell isolation and whole-cell patch-clamp recording to measure the electrical properties of the primary dissociated epithelial cells from the rat cauda epididymides. This protocol allows for investigation of the functional properties of primary epididymal epithelial cells to further elucidate the physiological role of the epididymis.
Streszczenie
The epididymis is an essential organ for sperm maturation and reproductive health. The epididymal epithelium consists of intricately connected cell types that are distinct not only in molecular and morphological features but also in physiological properties. These differences reflect their diverse functions, which together establish the necessary microenvironment for the post-testicular sperm development in the epididymal lumen. The understanding of the biophysical properties of the epididymal epithelial cells is critical for revealing their functions in sperm and reproductive health, under both physiological and pathophysiological conditions. While their functional properties have yet to be fully elucidated, the epididymal epithelial cells can be studied using the patch-clamp technique, a tool for measuring the cellular events and the membrane properties of single cells. Here, we describe the methods of cell isolation and whole-cell patch-clamp recording to measure the electrical properties of primary dissociated epithelial cells from the rat cauda epididymides.
Wprowadzenie
The epididymis in the male reproductive tract is an organ lined with a layer of mosaic epithelial cells. As in other epithelial tissues, the various cell types of the epididymal epithelium, including principal cells, clear cells, basal cells and cells from the immunological and lymphatic systems, work in a concerted manner to function as the barrier at the tubule frontline and as the supporting cells for sperm maturation and physiology1,2,3. Thus, these epithelial cells play an essential role in reproductive health.
Epithelial cells are generally regarded as non-excitable cells that are unable to generate all-or-none action potentials in response to depolarizing stimuli, due to a lack of voltage-gated Na+ or Ca2+ channels4,5. However, epithelial cells express unique sets of ion channels and transporters that regulate their specialized physiological roles, such as secretion and nutrient transportation6. Different epithelial cells therefore possess characteristic electrical properties. For example, the principal cells express the CFTR for fluid and chloride transportation and express the TRPV6 for calcium reabsorption, whereas the clear cells express the proton pump V-ATPase for luminal acidification1,7,8,9. Some transporters and ion channels that regulate the physiological features of the epididymal epithelial cells have been reported, but the functional properties of epididymal epithelial cells are largely not yet understood10,11,12,13.
Whole-cell patch-clamp recording is a well-established technique for examining the intrinsic properties of both excitable and non-excitable cells, and is particularly helpful for studying the functions of primarily dissociated cells in heterogeneous cell samples; the voltage-clamp is used for measuring the passive membrane properties and the ionic currents of single cells14,15. The passive membrane properties include input resistance and capacitance. The former parameter indicates the intrinsic membrane conductance, while the latter implies the surface area of the cell membrane (a phospholipid bilayer, where ion channels and transporters are located, that serves as a thin insulator separating extracellular and intracellular media). The membrane capacitance is directly proportional to the cell membrane's surface area. Together with the membrane resistance that is reflected by the input resistance, the membrane time constant, which indicates how fast the cell membrane potential responds to the flow of ion channel currents, can be determined. In this regard, by combining the current response characteristics from a series of voltage steps applied to the cells, the biophysical kinetics and properties of the cells are determined15,16,17,18.
In the present paper, we describe the procedures for isolating epithelial cells from the rat cauda epididymis and the steps for measuring the membrane properties of different cell types in the dissociated cell mixture using the whole-cell patch-clamp. We show that the epididymal principal cells exhibit distinct membrane electrophysiological properties and that the conductances can be readily identified from other cell types.
Access restricted. Please log in or start a trial to view this content.
Protokół
All animal experiments are carried out in accordance with the guidelines of the ShanghaiTech University Institutional Animal Care and Use Committee, which fulfill the local and international requirements.
1. Experimental Animals
- Use adult male Sprague-Dawley rats (~300-450 g) between 8-12 weeks old. At this age in the rats, the sperm have arrived in the cauda epididymides.
2. Isolation of Epithelial Cells from Rat Cauda Epididymides
NOTE: The following steps are performed under non-aseptic conditions unless otherwise stated.
- Preparation of dissection instruments and reagents
- Disinfect the dissection tools by immersion in 70% ethanol and let them air dry.
- Turn on the warming bath (32 °C); Prepare and pre-warm 500 mL of 1x Roswell Park Memorial Institute 1640 Medium (RPMI) supplemented with 1% (v/v) antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin final), and labeled as "RPMI (+P/S 1:100)". Perform this step in a clean air-flow controlled working station.
- Prepare and pre-warm 1x 500 mL Iscove's Modified Dulbecco's Medium (IMDM) containing non-essential amino acids (0.1 mM) and sodium pyruvate (1 mM), and supplemented with 5-α-dihydrotestosterone (1 nM), 10% fetal bovine serum, 1% (v/v) antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin final), and labeled as "Full-IMDM". Create 50 mL aliquots and seal with parafilm; store at 4 °C. Use aseptic conditions.
- Prepare a collagenase enzyme digestion solution by dissolving collagenase Type I and collagenase Type II in RPMI (+P/S 1:100) resulting in 1 mg/mL of each collagenase in the solution. Filter through a 0.22-µm membrane and mark as "Collagenase Solution". Keep at room temperature (RT) until use. Adjust the volume of the enzyme solution based on the weight of the enzyme; the minimal volume required for both cauda epididymides from a single rat is 2 mL.
- Fill a 35 mm dish with RPMI (+P/S 1:100).
- Dissection of rat cauda epididymides
- Sacrifice the animal by either using sodium pentobarbital 85 mg/kg i.p. or using an isoflurane chamber until the animal does not respond to tail-pinching stimulation; follow by cervical dislocation.
- Disinfect the lower abdomen by wiping with 70% ethanol, gently push the two testes down to the lower abdomen, and then open the lower abdomen near the scrotum.
- Pick up the epididymal fat, dissect out the whole reproductive organs (testes, epididymides, and vas deferens) and immerse in the dish with RPMI (+P/S 1:100).
- Transfer the reproductive organs in the dish with RPMI (+P/S 1:100) to an aseptic working station.
- Dissect out the cauda epididymides from the connective and fatty tissues and the epididymal capsule. Place one epididymis with ~0.2 mL of Collagenase Solution in a 1.5 mL tube.Perform this step in a clean air-flow controlled working station.
- Dissociation of single cells from rat cauda epididymides
- Cut the epididymides in the Collagenase Solution using fine scissors until the tissue becomes a paste-like fluid. Rinse the scissors gently with the rest of (~0.8 mL) enzyme solution in the 1.5 mL tube.
- Place the tube on a metal thermomixer for 30 min at 37 °C with a shaking speed of 1,000 rpm.
- Centrifuge the enzyme-tissue mixture at 30 x g at RT for 3 min and decant the sticky supernatant that contains mostly the sperm.
- Resuspend the pellet in 1 mL Full-IMDM to quench all the enzymatic activity. Transfer the cell suspension to a 50 mL tube containing 49 mL RPMI (+P/S 1:100).
NOTE: Optionally, filter the cell suspension through a 100 µm mesh membrane with constant trituration to avoid large, cell aggregates. However, do not use a mesh filter if cell suspension is needed for growing cell monolayers. - Centrifuge the cellular mixture at 30 x g at RT for 10 min; decant the supernatant.
- Resuspend the pellet in 1 mL Full-IMDM with gentle trituration for at least 5 min to dissociate single cells from the enzymatic treated epididymal tissue mixtures.
- Separation of epithelial cells from other cells under aseptic conditions
- Culture the cell suspension on a 10 cm Petri dish containing Full-IMDM for at least 8 h or overnight, in an incubator at 32 °C in 5% CO2.
- Prepare sterile coverslips in advance by immersion in 100% alcohol. Air dry and dip in a small volume of the culture medium. Place the coverslips in 6 cm culture dishes or in single wells of a 24-well plate.
- Next morning, harvest the dissociated epithelial cells by gently collecting the cell suspension from the Petri dish, which consists mostly of epithelial cells. Centrifuge the cell suspension at 30 x g at RT for 5 min, and then decant the supernatant.
- Resuspend the cell pellet in ~2 mL Full-IMDM.
- Seed 0.2 mL of the harvested cell suspension onto the center of each sterile coverslip.
- Let the cell suspension settle in the liquid droplet for at least 10 min to allow cells to loosely adhere to the glass coverslips. Carefully add 1 mL Full-IMDM at the edge of 10 cm dish, or 0.3 mL Full-IMDM to each well of a 24-well plate; do not disturb cells.
- Keep the isolated single cells on coverslips in the incubator at 32 °C in 5% CO2 until the patch-clamp experiments.
3. Recording Solutions and Micropipettes
NOTE: For the patch-clamp experiments, use the best quality chemicals and solutions.
- Preparation of stock solutions
- Autoclave all the bottles for stock storage and filter all the stock solutions (except the corrosive solutions) and filter through 0.22-µm membranes before use.
- Prepare all stock solutions in advance at RT, and store at 4 oC: 5 M NaCl; 1 M KCl; 100 mM MgCl2; 100 mM CaCl2; 200 mM NaH2PO4; 100 mM EGTA (pH 7.0 with KOH). Handle 5 M NaOH, 1 M HCl and 1 M KOH stocks as corrosive solutions.
- Preparation of standard external recording physiological salt solution (PSS)
- Warm the stock solutions to RT on the morning of the patch-clamp recording.
- Pipette the ingredients from each stock according to the desired final volume, except CaCl2, e.g. for preparing 500 mL of PSS: 140 mM NaCl = 14 mL of 5M; 5 mM KCl = 2.5 mL of 1M; 1.2 mM MgCl2 = 6 mL of 100 mM; 1.2 mM NaH2PO4 = 3 mL of 200 mM.
- Add double-distilled water (ddH2O) to the final volume of 400 mL and equilibrate.
- Weigh 0.9 g glucose and 1.19 g HEPES and dissolve completely in the solution mixture.
- Add the CaCl2 stock (2.5 mM = 12.5 mL of 100 mM) with stirring.
- Add up to 99% of final volume.
- Adjust the pH to 7.4 using NaOH or HCl.
- Check the osmolarity and adjust using 5 M NaCl or glucose, if necessary.
- Add ddH2O to the final volume of 500 mL in a cylinder.
- Preparation of micropipette internal solutions (low EGTA K+ -based solutions)
- Weigh or pipette the correct volume of the reagents from each stock according to the desired final volume and concentration, e.g. for preparing 50 mL low EGTA K+-based intracellular solution to a volume of ~ 30 mL ddH2O: 100 mM K-gluconate = 1.17 g; 35 mM KCl = 1.75 mL of 1 M; 2 mM MgCl2 = 1 mL of 100 mM; 0.1 mM EGTA = 0.05 mL of 100 mM; 10 mM HEPES = 0.072 g.
- Add enough water for ~ 95% of final volume and allow the solution to equilibrate at RT. Make sure that the solution is clear.
- While constantly stirring the solution, adjust the pH to 7.2 using KOH.
- Weigh and add 0.078 g Mg-ATP to the solution until it is dissolved completely.
- Place the solution on ice and use a small aliquot for the measurement of osmolarity; typically, the solutions measures ~290 mOsmol and does not need adjustment. If the osmolarity differs significantly from 280-295 mOsmol, prepare a new solution.
- Add ddH2O to final volume.
- Divide the solution into 500 µL aliquots, filter with a 0.2 µm syringe filter, tightly seal and immediately store at ≤ -20 °C.
- On the date of the patch-clamp experiment, thaw one aliquot of intracellular solution on ice and keep chilled during the patch-clamp experiment to prevent degradation.
- Pull the patch pipettes from glass capillaries (following pipette puller user's manual) to obtain micropipette sizes with resistance of 5-10 M when filled with intracellular solution.
4. Setting up the Patch-Clamp Experiment and Establishing Whole-Cell Configuration with Cells
- Setting up the patch-clamp experiment
- Turn on the patch-clamp set up (computer, computer-controlled amplifier, digitizer, etc.)
- Open the patch-clamp software (e.g. AXON pCLAMP10 or HEKA PatchMaster) and set up the protocols for electrophysiological recordings. Set the filter for the signal to low pass at 1-3 kHz and the digitizer at 10-20 kHz.
- Turn on the camera, micromanipulator and light source.
- When the computer-controlled amplifier is on, ground the body of experimenter by touching with hands the patch-clamp rig that has been grounded, before touching the headstage, in order to protect it from electrical shocks.
- Transfer the culturing epithelial cells on the glass coverslip to the recording chamber filled with ~1 mL of standard PSS at RT. Carefully change the bathing PSS at least two times using a pipette before any patch-clamp experiments.
NOTE: Optionally, fill the perfusion system with standard PSS or another external solution according to the planned experiment. Perfuse the microscope-mounted recording chamber (RC-26G or RC-26GLP) with PSS a few times at a speed of ~2 mL/min before the start off the patch-clamp experiments. Make sure that there are no air bubbles trapped along the perfusion system. - View and select the cells under an inverted microscope using 10X and 40X objectives equipped with a differential interference contrast optical system. Look for large single isolated cells for recording. Identify the isolated epididymal epithelial cells by their spherical shape with rough microvilli on one end of the membranes and polarized distribution of intracellular contents (Figure 1).
- Using a 1 mL syringe (a home-made nonmetallic microsyringe needle), fill a micropipette with the internal solution (low EGTA K+-based solution, see step 3.3). Make sure that there are no air bubbles in the micropipette, which can increase the resistance of the micropipette. Use enough solution so that the internal solution submerges the chloride-coated silver wire electrode within the micropipette holder.
- Mount the micropipette in the electrode holder, and apply a low positive pressure (~0.2 mL syringe volume). Keep the low positive pressure continuous until touching the cell membrane in the later steps.
- Establishing whole-cell configuration with cells for recordings
- Immerse the pipette into the bath solution at the highest speed of the micromanipulator. Find the pipette tip in the screen connected to the digital camera; slow down the micromanipulator speed to the medium-high mode.
- Quickly check the micropipette resistance (5-10 MΩ) using the data acquisition interface command (e.g. "Membrane Test" in the AXON system) by applying a voltage step (e.g. 5 mV for 100 ms) generated from the computer-controlled amplifier. Change to a new micropipette if the resistance is significantly out of this range.
- Start to move down the objective mounted on the microscope; gradually guide the micropipette toward the selected cell. Always lower the objective first, and then lower the micropipette to the plane of focus, untilthe micropipette is above the center surface of the selected cell.
- Cancel the liquid junction potential between the pipette and bath solutions to zero using the "pipette offset" command in the commander interface of software.
- Set the computer-controlled amplifier commander to the voltage-clamp and the membrane test to the "Bath" mode.
- Fine focus for a clearer view of the cell, then gradually lower the micropipette using the micromanipulator at the low-medium speed.
- When the micropipette is close to the cell (demonstrated by a decreased current when triggered by the membrane test command), remove the low positive pressure immediately and apply a weak negative pressure (0.1 mL syringe volume) to form the gigaseal (>1 GΩ).
- Monitor the resistance with the membrane test. If the resistance is >500 MΩ but <1 GΩ, apply a negative potential (usually as the holding potential which is set to -60 mV), which can help form the gigaseal. Compensate the transient capacitive current of the micropipette.
- If the seal is >1 GΩ and stable (as shown in the software interface), apply a brief and strong suction in order to break the cell membrane. Do not apply compensation for the series resistance and the cell capacitance.
- Immediately after achieving a successful whole-cell configuration, apply a 10 mV hyperpolarizing step (5-traces with minimal time intervals, 20 ms duration, signal sample at 20 kHz) from a holding potential of -60 mV.
- Switch the voltage-mode to the zero-current mode and mark down the readings from the software interface or perform a gap-free recording (10-60 s) for the membrane potential readings of the cell.
- Quickly switch back to and remain in the voltage-mode and apply the voltage protocols according to the planned experiments and measure the current responses. Subtraction is not applied to the intrinsic leak current during the recordings.
- Monitor the stability of the responses during the recordings, or the different parameters of the cell. For example, use the "Membrane Test" command interface to check the input resistance (Ri), the series resistance (Rs) and the cell membrane capacitance (Cm) in the membrane test at the "Cell" mode during the switch of protocols.
NOTE: Sudden drops in input resistance is usually indicative of a loose patch, and a dramatic increase in the series resistance may be indicative of micropipette tip clogging by the intracellular organelles or membrane fragments. During recording, regularly monitor the micropipette location to check if drift occurs, which may lead to the loss of the patch. If drift is a problem, carefully lift together the micropipette and the patching cell away from the bottom of recording chamber. This step may sometimes lead to the loss of the patch, in which case, it is necessary to repeat the whole-cell patch-clamp procedure.
5. Analysis of Passive Electrophysiological Properties of Cells
- After obtaining the data from the patch-clamp experiments, open the gap-free data in the Clampfit software and measure the mean value for the resting membrane potential for each cell. Alternatively, use the value as marked down from the zero-current voltage at the current mode. Correct the values with the liquid junction potential (12.4 mV in this study).
- Open the data from the 10-mV hyperpolarizing step (ΔV) obtained from a cell, measure the current difference before and during the step (ΔIstep), and calculate the input resistance (Ri) using the equation as:

- Calculate the cell capacitance (Cm, in unit of pF) (use the same current data from the 10-mV hyperpolarizing step by integrating the total area under the membrane capacitor current during the initial transient decay current raised upon the voltage step trigger), to obtain the value of the total accumulated charge (Q, in unit of pA•ms) of the cell. Use the following equation:
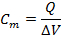
- Calculate the value of the series resistance (Rs) for each cell by fitting the initial transient current from the 10-mV negative step with a standard exponential algorithm to obtain the time constant decay current (T, in unit of ms) and use the following equation:

Access restricted. Please log in or start a trial to view this content.
Wyniki
The described enzymatic digestion procedure for the isolation of epithelial cells from the rat cauda epididymides is a modified protocol from our previous studies9,12. This method produces a mixture of single cells with over 90% viability and without surface blisters or swollen cell volume. The heterogeneous cell mixture consists mainly of principal cells, clear cells and basal cells, as we have described previously
Access restricted. Please log in or start a trial to view this content.
Dyskusje
In this protocol, the enzymatic dispersion of the rat cauda epididymides consistently yielded healthy epithelial cells. The quality of the epididymal epithelial cells for the patch-clamp experiments is dependent on a few critical steps in the protocol. For instance, the centrifugation of the cell mixture at a low centrifugal force (30 x g) is important for removing the spermatozoa and the epididymal luminal content; the epididymal epithelial cells become unhealthy in the presence of the spermatozoa in the cell culture. I...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We thank Dr. Christopher Antos for helpful comments on the text. This work was supported by start-up funding from ShanghaiTech University awarded to Winnie Shum and by the funding from the National Natural Science Foundation of China (NNSFC no. 31471370).
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Instrument of AXON system | |||
| Computer controlled amplifier | Molecular Devices - Axon | Multiclamp 700B patch-clamp amplifier | |
| Digital Acquisition system | Molecular Devices - Axon | Digidata 1550 converter | |
| Microscope | Olympus | BX-61WI | |
| Micromanipulator | Sutter Instruments | MPC-325 | |
| Recording chamber and in-line Heater | Warner Instruments | TC-324C | |
| Instrument of HEKA system | |||
| Patch Clamp amplifier | Harvard Bioscience - HEKA | EPC-10 USB double | |
| Microscope | Olympus | IX73 | |
| Micromanipulator | Sutter Instruments | MPC-325 | |
| Recording chamber and in-line Heater | Warner Instruments | TC-324C | |
| Other Instrument | |||
| Micropipette Puller | Sutter Instrument | P-1000 | |
| Recording Chamber | Warner Instruments | RC-26G or homemade chamber | |
| Borosilicate capillary glass with filament | Sutter Instrument / Harvard Apparatus | BF150-86-10 | |
| Vibration isolation table | TMC | 63544 | |
| Digital Camare | HAMAMASTU | ORCA-Flash4.0 V2 C11440-22CU | |
| Reagents for isolation | |||
| RPMI 1640 medium | Gibco | 22400089 | |
| Penicillin/Streptomycin | Gibca | 15140112 | |
| IMDM | ATCC | 30-2005 | |
| IMDM | Gibco | C12440500BT | |
| Collagenase I | Sigma | C0130 | |
| Collagenase II | Sigma | C6885 | |
| 5-α-dihydrotestosterone | Medchemexpress | HY-A0120 | |
| Fetal bovine serum | capricorn | FBS-12A | |
| Micropipette internal solutions (K+-based solution) (pH 7.2, 280-295 mOsm) | |||
| KCl, 35mM | Sigma/various | V900068 | |
| MgCl2 · 6H2O, 2mM | Sigma/various | M2393 | |
| EGTA, 0.1mM | Sigma/various | E4378 | |
| HEPES, 10mM | Sigma/various | V900477 | |
| K-gluconate, 100mM | Sigma/various | P-1847 | |
| Mg-ATP, 3mM | Sigma/Various | A9187 | |
| The standard external recording physiological salt solution (PSS) (pH 7.4, 300-310 mOsm) | |||
| NaCl, 140mM | Sigma/various | V900058 | |
| KCl, 4.7mM | Sigma/various | V900068 | |
| CaCl2, 2.5mM | Sigma/various | V900266 | |
| MgCl2 · 6H2O, 1.2mM | Sigma/various | M2393 | |
| NaH2PO4, 1.2mM | Sigma/various | V900060 | |
| HEPES, 10mM | Sigma/various | V900477 | |
| Glucose, 10mM | Sigma/various | V900392 | |
| For pH adjustment | |||
| NaOH | Sigma/various | V900797 | Purity >=97% |
| KOH | Sigma/various | 60371 | Purity >=99.99% |
Odniesienia
- Shum, W. W. C., Ruan, Y. C., Da Silva, N., Breton, S. Establishment of Cell-Cell Cross Talk in the Epididymis: Control of Luminal Acidification. J Androl. 32 (6), 576-586 (2011).
- Robaire, B., Hinton, B. T. Knobil and Neill's Physiology of Reproduction. Plant, T. M., Zeleznik, A. J. , Elsevier. 691-771 (2015).
- Da Silva, N., et al. A dense network of dendritic cells populates the murine epididymis. Reproduction. 141 (5), 653-663 (2011).
- Kolb, H. A. Special Issue on Ionic Channels II. , Springer. 51-91 (1990).
- Clapham, D. E. Calcium Signaling. Cell. 80 (2), 259-268 (1995).
- Frizzell, R. A., Hanrahan, J. W. Physiology of Epithelial Chloride and Fluid Secretion. Cold Spr Harb Pers Med. 2 (6), (2012).
- Wong, P. Y. D. CFTR gene and male fertility. Mol Hum Reprod. 4 (2), 107-110 (1998).
- Shum, W. W. C., et al. Transepithelial Projections from Basal Cells Are Luminal Sensors in Pseudostratified Epithelia. Cell. 135 (6), 1108-1117 (2008).
- Gao, D. Y., et al. Coupling of TRPV6 and TMEM16A in epithelial principal cells of the rat epididymis. J Gen Physiol. 148 (2), 161-182 (2016).
- Huang, S. J., et al. Electrophysiological Studies of Anion Secretion in Cultured Human Epididymal Cells. J Physiol. 455, 455-469 (1992).
- Chan, H. C., Fu, W. O., Chung, Y. W., Chan, P. S. F., Wong, P. Y. D. An Atp-Activated Cation Conductance in Human Epididymal Cells. Biol Repro. 52 (3), 645-652 (1995).
- Cheung, K. H., et al. Cell-cell interaction underlies formation of fluid in the male reproductive tract of the rat. J Gen Physiol. 125 (5), 443-454 (2005).
- Pastor-Soler, N., Pietrement, C., Breton, S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiol. 20, 417-428 (2005).
- Evans, A. M., Osipenko, O. N., Haworth, S. G., Gurney, A. M. Resting potentials and potassium currents during development of pulmonary artery smooth muscle cells. Ame J Physiol-Heart Circ Physiol. 275 (3), 887-899 (1998).
- Golowasch, J., et al. Membrane Capacitance Measurements Revisited: Dependence of Capacitance Value on Measurement Method in Nonisopotential Neurons. J Neurophysiol. 102 (4), 2161-2175 (2009).
- Cole, K. S. Membranes, Ions and Impulses. A Chapter of Classical Biophysics. , University of California Press. Berkeley, Calif. (1968).
- Lo, C. M., Keese, C. R., Giaever, I. Impedance analysis of MDCK cells measured by electric cell-substrate impedance sensing. Biophys J. 69, 2800-2807 (1995).
- Solsona, C., Innocenti, B., Fernandez, J. M. Regulation of exocytotic fusion by cell inflation. Biophys J. 74 (2), 1061-1073 (1998).
- Robinson, D. W., Cameron, W. E. Time-dependent changes in input resistance of rat hypoglossal motoneurons associated with whole-cell recording. J Neurophysiol. 83 (5), 3160-3164 (2000).
- Sontheimer, H. Neuro Methods: Patch-Clamp Applications and Protocols. Boulton, A. A., Baker, G. B., Walz, W. , Humana Press. New Jersey. (1995).
- Cheung, Y. M., Hwang, J. C., Wong, P. Y. Epithelial membrane potentials of the epididymis in rats [proceedings]. J Physiol. 263 (2), 280(1976).
- Lybaert, P., et al. KATP channel subunits are expressed in the epididymal epithelium in several mammalian species. Biol Reprod. 79 (2), 253-261 (2008).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone