Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Predicting Gene Silencing Through the Spatiotemporal Control of siRNA Release from Photo-responsive Polymeric Nanocarriers
W tym Artykule
Podsumowanie
We present a novel method that uses photo-responsive block copolymers for more efficient spatiotemporal control of gene silencing with no detectable off-target effects. Additionally, changes in gene expression can be predicted using straightforward siRNA release assays and simple kinetic modeling.
Streszczenie
New materials and methods are needed to better control the binding vs. release of nucleic acids for a wide range of applications that require the precise regulation of gene activity. In particular, novel stimuli-responsive materials with improved spatiotemporal control over gene expression would unlock translatable platforms in drug discovery and regenerative medicine technologies. Furthermore, an enhanced ability to control nucleic acid release from materials would enable the development of streamlined methods to predict nanocarrier efficacy a priori, leading to expedited screening of delivery vehicles. Herein, we present a protocol for predicting gene silencing efficiencies and achieving spatiotemporal control over gene expression through a modular photo-responsive nanocarrier system. Small interfering RNA (siRNA) is complexed with mPEG-b-poly(5-(3-(amino)propoxy)-2-nitrobenzyl methacrylate) (mPEG-b-P(APNBMA)) polymers to form stable nanocarriers that can be controlled with light to facilitate tunable, on/off siRNA release. We outline two complementary assays employing fluorescence correlation spectroscopy and gel electrophoresis for the accurate quantification of siRNA release from solutions mimicking intracellular environments. Information gained from these assays was incorporated into a simple RNA interference (RNAi) kinetic model to predict the dynamic silencing responses to various photo-stimulus conditions. In turn, these optimized irradiation conditions allowed refinement of a new protocol for spatiotemporally controlling gene silencing. This method can generate cellular patterns in gene expression with cell-to-cell resolution and no detectable off-target effects. Taken together, our approach offers an easy-to-use method for predicting dynamic changes in gene expression and precisely controlling siRNA activity in space and time. This set of assays can be readily adapted to test a wide variety of other stimuli-responsive systems in order to address key challenges pertinent to a multitude of applications in biomedical research and medicine.
Wprowadzenie
Small interfering RNAs (siRNAs) mediate post-transcriptional gene silencing through a catalytic RNAi pathway that is highly specific, potent, and tailorable to virtually any target gene1. These promising characteristics have enabled siRNA therapeutics to advance in human clinical trials for the treatment of numerous diseases, including metastatic melanoma and hemophilia2,3. However, significant delivery issues persist that have hindered translation4. In particular, delivery vehicles must remain stable and protect siRNAs from extracellular degradation, yet also release the payload into the cytoplasm5. Furthermore, many RNAi applications require improved methods to regulate gene silencing in space and time6, which will reduce side effects in siRNA therapeutics7 and enable transformative advances in applications ranging from cell microarrays for drug discovery8 to modulation of cell responses in regenerative scaffolds9. These challenges highlight the need for new materials and methods to better control binding vs. release in siRNA nanocarriers.
One of the most promising strategies for controlling siRNA release and enhancing spatiotemporal regulation is the use of stimuli-responsive materials10. For example, a wide variety of biomaterials have been engineered with changeable nucleic acid binding affinity in response to altered redox potential or pH, or applied magnetic fields, ultrasound, or light11. Although many of these systems demonstrate improved control over nucleic acid activity, the use of light as a trigger is particularly advantageous due to its instantaneous temporal response, precise spatial resolution, and ease of tunability12. Moreover, the potential of photo-sensitive technologies for regulating gene expression has been demonstrated by state-of-the-art inducible promoter and optogenetic regulator systems; however, these systems suffer from numerous challenges including limited capacities to regulate endogenous genes, safety concerns such as immunogenicity, and difficulties in delivering multi-component assemblies13,14,15. Photo-responsive siRNA nanocarriers are ideally suited to overcome these drawbacks and provide a simpler and more robust approach to spatiotemporally modulate gene expression16,17,18. Unfortunately, methods to accurately predict the resulting protein knockdown response remain elusive.
A key challenge is that quantitative evaluations of siRNA release are rare19,20, and even when these evaluations are performed, they have not been coupled to analyses of siRNA/protein turnover dynamics. Both the amount of siRNA released and its persistence/lifetime are important determinants of the resulting gene silencing dynamics; hence, a lack of such information is a major disconnect that precludes accurate prediction of dose-response in RNAi21. Addressing this challenge would expedite the formulation of the appropriate structure-function relationships in nanocarriers and better inform biomaterial designs22. Furthermore, such approaches would enable development of more effective siRNA dosing protocols. In an attempt to understand the dynamic silencing response, several groups have investigated mathematical models of RNAi23,24,25. These frameworks were successful in providing insights into siRNA-mediated changes in gene expression and identifying rate-limiting steps26. However, these models have been applied only to commercial gene delivery systems (e.g., Lipofectamine and polyethylenimine (PEI)) that are not capable of controlled siRNA release, and the complexity of the models has severely limited their utility27. These shortcomings highlight an unmet need for new materials capable of precisely tunable siRNA release combined with streamlined and easy-to-use predictive kinetic models.
Our method addresses all of these challenges through the integration of a light-sensitive nanocarrier platform with coupled methods to quantify free siRNA and model RNAi dynamics. In particular, our platform's precisely controlled siRNA release28 is monitored by two complementary methods for accurately quantifying encapsulated vs. unbound siRNA. The experimental data from these assays are entered into a simple kinetic model to predict gene silencing efficiencies a priori29. Finally, the on/off nature of the nanocarriers is easily exploited to generate cell patterns in gene expression with spatial control on the cellular length scale. Thus, this method provides an easily adaptable method to control and predict gene silencing in a variety of applications that would benefit from spatiotemporal regulation of cell behavior.
Protokół
1. Formulation of siRNA Nanocarriers
- Prepare separate solutions of siRNA and mPEG-b-P(APNBMA) with equal volumes diluted in 20 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer at pH 6.0.
- Add siRNA at a concentration of 32 µg/mL to 20 mM HEPES solution.
NOTE: The siRNA was a non-targeted, universal negative control sequence; however, the siRNA can be designed to target any gene of interest. - Dissolve mPEG-b-P(APNBMA) polymers into a 20 mM HEPES solution. Add an appropriate amount of mPEG-b-P(APNBMA) to make a 220 µg/mL solution so that the N/P ratio (N, amine groups on mPEG-b-P(APNBMA); P, phosphate groups on siRNA) is 4.
NOTE: The synthetic protocol for the mPEG-b-P(APNBMA) polymers is reported elsewhere30.
- Add siRNA at a concentration of 32 µg/mL to 20 mM HEPES solution.
- Add the mPEG-b-P(APNBMA) solution dropwise to an equal volume of the siRNA solution while gently mixing on a vortex machine. Continue to vortex for 30 s following polymer addition. Incubate the samples in the dark at room temperature for 30 min.
2. Measuring siRNA Release Using Gel Electrophoresis
- Formulate the nanocarriers according to steps 1.1-1.2, and scale the volumes to accommodate the number of samples desired.
- Mix the nanocarrier with sodium dodecyl sulfate (SDS).
- Prepare a 1 mg/mL solution of SDS in water. Aliquot out the amount of SDS solution needed to produce solutions with an S/P ratio (S, sulfate groups on SDS; P, phosphate groups on siRNA) of 15.
NOTE: If the polyplex solution contains 1 µg of siRNA, 13 µg of SDS must be added to achieve an S/P ratio of 15. - Add the SDS solution to each nanocarrier solution dropwise while gently mixing on a vortex machine. Continue to vortex for 30 s following SDS addition.
- Centrifuge the samples at 3,000 x g for 5 s. Incubate the samples in the dark at room temperature for 30 min.
- Prepare a 1 mg/mL solution of SDS in water. Aliquot out the amount of SDS solution needed to produce solutions with an S/P ratio (S, sulfate groups on SDS; P, phosphate groups on siRNA) of 15.
- Calibrate and set a UV laser with a 365 nm filter to an intensity of 200 W/m. Ensure that the light intensity is measured from the location at which the bottom of the sample solution will be seated.
- Load the nanocarrier/SDS solution into a glass chamber comprised of glass slides separated by a rubber gasket.
- Pre-wash glass slides in a 7:3 (v/v) ethanol/water solution in water and dry completely. Cut a hole (~2 x 3 cm rectangle) into a rubber gasket. Place the rubber gasket onto a glass slide.
- Pipette the nanocarrier/SDS solution onto the glass slide inside the hole of the rubber gasket. Load an excess of solution (20 µL in surplus) onto the glass slide while avoiding contact with the rubber gasket.
NOTE: Some liquid will be lost during the subsequent steps. - Place the second glass slide on top of the slide-gasket. To avoid air bubble generation, place one end of the slide down first and then slowly lower the other end.
- Attach binder clips to each side of the glass chamber to hold it closed.
- Irradiate the samples for the desired length of time (e.g., 0-60 min) using the UV laser with a 365 nm filter at an intensity of 200 W/m. Remove the binder clips and open the chamber.
- Pipette 25 µL of the irradiated nanocarrier/SDS samples into a microcentrifuge tube. Incubate the solutions in the dark at room temperature for 30 min.
- Prepare a 2 wt% agarose gel pre-stained with 0.5 µg/mL ethidium bromide in Tris/Borate/EDTA (TBE) buffered solution according to standard protocols31. Prepare a loading buffer comprised of 3:7 (v/v) glycerol/water.
- Add 5 µL of the loading buffer solution to 25 µL of each nanocarrier/SDS sample. Incubate the samples in the dark at room temperature for 10 min.
- Load 30 µL of each nanocarrier/SDS sample into the 2% agarose gel. Run the gel in the dark at 100 V for 30 min. Image the gel using a gel imaging system with ethidium bromide filters. Save the gel image files and proceed to step 2.10 for band intensity quantification. Ensure that the band intensities are bright enough to clearly visualize but not too bright that the signals are saturated.
- Quantify the band intensities using publicly available ImageJ software32.
- Using the ROI tool of ImageJ, determine the fluorescence intensity of the free siRNA bands in each lane by drawing a rectangle around each band. Plot the intensity curves of each lane, and integrate the area under the curves by drawing a horizontal line across the intensity curves and clicking the tracing wand inside the enclosed areas.
- Compute the relative intensity of each lane by dividing the area under the curve of each sample by the area under the curve of the siRNA positive control (no mPEG-b-P(APNBMA) added and no SDS added). Report the percentage of siRNA released as the normalized band intensity of each sample.
3. Measuring siRNA Release Using Fluorescence Correlation Spectroscopy (FCS)
- Obtain siRNA labelled with a single fluorophore at the 5' end of the sense strand.
NOTE: The siRNA can be purchased pre-annealed with the labels conjugated in the desired location. The fluorophore should be photo-stable and absorb/emit between 450 and 750 nm to avoid UV light quenching and energy transfer with mPEG-b-P(APNBMA). - Formulate the nanocarriers according to steps 1.1-1.2 using the labelled siRNA. Scale the volumes to accommodate the number of samples desired.
- Incubate the solutions in SDS and irradiate for the desired length of time according to steps 2.2-2.6.
- Preparation of FCS sample chamber.
- Wash a glass slide with a 7:3 (v/v) ethanol/water solution and completely dry the glass using a wipe and an air stream.
- Remove the pieces of paper from a double-sided adhesive spacer to expose the double-sided adhesive spacer. Attach the spacer to a glass coverslip.
- Pipette the nanocarrier/SDS solution onto the cover slip in the middle of the hole from the adhesive spacer.
- Place the glass slide on top of the coverslip. Push on the glass slide to ensure that the glass slide and coverslip are well attached and form a seal.
- Use a confocal microscope for FCS measurements33. Use a 40X water immersion apochromat objective with a numerical aperture of 1.2. Use the appropriate excitation laser channel (488 nm) to collect at least 30 measurements of 10 s each per sample34. Ensure that the laser intensity and detector alignment remain the same for each sample.
- In addition to the experimental samples, measure controls including: a blank sample without labelled siRNA; and a free siRNA sample with labelled siRNA but no mPEG-b-P(APNBMA).
- Analyze the data using FCS-specific software. Identify the baseline count rate of each sample by determining the stable count rate during a time when no nanocarriers are passing through the confocal volume29.
- Subtract the count rate of the blank sample from every sample baseline count rate value. Normalize the resulting values to the free siRNA control to calculate the percent of free siRNA35.
4. Kinetic Modeling to Predict Gene Silencing
- Create scripts in a mathematical programming language using the simple set of ordinary differential equations to predict gene silencing29.
NOTE: Scripts can be made available upon request.- Write the set of ordinary differential equations as:
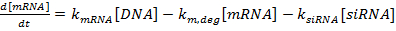 (1)
(1)
 (2)
(2)
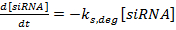 (3)
(3)
NOTE: For equations 1−3, the terms kmRNA, ksiRNA, and kprot are the rate constants for the production of mRNA, siRNA, and protein, respectively. The terms km,deg, ks,deg, and kp,deg are the rate constants for the degradation of mRNA, siRNA, and protein, respectively. Degradation rate constants are computed on the basis of the component half-lives, and production rate constants are fit to ensure that mRNA and protein steady-state values are reached in the absence of siRNA.- Determine the half-lives of the mRNA and protein for the gene(s) of interest, either experimentally as described in reference 36 or from the literature (see the Discussion). Also, determine the doubling time for the cell line. Input these values into the appropriate degradation rate expressions.
- Tune the production rate constants so that gene expression levels remain steady at a normalized value of 100 if no siRNA is introduced. Specifically, set [siRNA] to zero and vary the values of the kmRNA, ksiRNA, and kprot production rate constants until [mRNA] and [protein] remain within 1% of the initial normalized value of 100% for the duration of the simulation.
- Using the relative amounts of siRNA released from the previously described gel electrophoresis and FCS assays as estimates, adjust the initial relative concentration of siRNA in the script. Specifically, vary [siRNA] to be proportional to the relative amount of released siRNA, with a value of 100 corresponding to the maximum amount29.
- Write the set of ordinary differential equations as:
5. Cell Culture and In Vitro siRNA Delivery
- Culture NIH/3T3 murine embryonic fibroblasts according to the protocols from the supplier.
- Grow the cells in growth medium (Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin). Maintain the cells at 37 °C in a humidified environment with 5% CO2.
- Seed the cells in 6-well tissue culture treated plates.
- Follow the recommended subculturing procedure from the supplier. Count the cells using a hemocytometer. Dilute the cells in supplemented growth media to a concentration of 75,000 cells/mL.
- Add 2 mL of cell suspension (75,000 cells/mL) to each well of the 6-well plate. Let the cells adhere and recover for 24 h in the incubator.
- Prepare the cells for transfection by washing with phosphate-buffered saline (PBS) and adding 1.5 mL of serum- and antibiotic-free transfection medium (see the Table of Materials) to each well.
- Formulate the siRNA nanocarriers according to steps 1.1-1.2. Add 25 µL of nanocarrier solution containing 30 pmol of siRNA to each well. Gently pipette the media up and down to mix. Place the cells in the incubator for 3 h.
- Remove the transfection media and wash each well with PBS. Add 1 mL of supplemented growth media and place the cells in the incubator to recover for 30 min.
- To prepare the cells for treatment with a photo-stimulus, remove the supplemented growth media. Add 1 mL of transfection media (without phenol red) to each well.
NOTE: Ensure that the transfection media does not contain phenol red. - Calibrate and set a UV laser with a 365 nm filter to an intensity of 200 W/m. Ensure that the light intensity is measured from the location at which the bottom of the cell plate will be seated.
- Place the cells on a hot plate set to 37 °C. Remove the plate cover of the cells. Irradiate the cells from above the plate for the desired time (up to 20 min) using the UV laser with a 365 nm filter at an intensity of 200 W m-2.
- Remove the transfection media and add 2 mL of supplemented growth media. Place in incubator until further analysis (e.g., 24 h for qPCR and 48 h for Western blotting).
- Measure changes in gene expression using a variety of techniques such as Western blotting37 and qPCR.38 For genes with visible signals, such as GFP, use fluorescence microscopy29.
NOTE: These techniques are suggested due to their ease of use and accuracy in quantifying gene expression
- Measure changes in gene expression using a variety of techniques such as Western blotting37 and qPCR.38 For genes with visible signals, such as GFP, use fluorescence microscopy29.
6. Controlling Gene Silencing in a Spatiotemporal Manner
- Culture, seed, and transfect cells according to steps 5.1-5.7.
- Prepare a photomask that completely blocks 365 nm light and minimizes reflections.
NOTE: In this case, 10 x 10 cm pieces of aluminum foil and black construction paper were used to block the light and to reduce reflections, respectively. The aluminum foil and construction paper were glued together to form a single unit.- Cut/punch/machine the desired shape into the photomask. For example, use a sharp-edged blade and a hole-puncher to form a straight-line pattern (~5 cm long) and a circular pattern (~7 mm diameter) in the photomask, respectively.
- Glue the photomask to the bottom of the 6-well plate with the pattern centered under the well containing the cells with the anti-reflective side (e.g., black construction paper) facing the plate. Ensure that the glue is not placed near the edge (within ~3 mm) of the pattern.
- Set up two ring stands approximately 25 cm apart and attach a platform to each ring stand so that the platforms are of equal height. Suspend the cell plate between the two stands by resting the plate on top of the platforms. Ensure that the plate is level.
- Irradiate the cells from below the sample for the desired time (up to 20 min) using the UV laser with a 365 nm filter at an intensity of 200 W/m2.
- Remove the transfection media and add 2 mL of supplemented growth media. Place in incubator to recover for at least 24 h. Image the cells using fluorescence microscopy as described29.
Wyniki
Following the formulation of the nanocarriers, siRNA release assays were conducted to inform the irradiation conditions to be used in the in vitro transfections. Various dosages of light were applied to determine the percent of siRNA that was released. The first assay used gel electrophoresis to separate the free siRNA molecules from the siRNA molecules still complexed/associated with the polymer. Nanocarriers that were not treated with light remained stable and did not release a...
Dyskusje
There are a few steps in the method that are particularly critical. When formulating the nanocarriers, the order of component addition and mixing speed are two important parameters influencing efficacy39. This protocol requires that the cationic component, mPEG-b-P(APNBMA), is added to the anionic component, siRNA, in a dropwise fashion while vortexing. Depending on the total formulation volume, this mixing process takes 3-6 s. To test if the nanocarriers were formed properly, measure the...
Ujawnienia
The authors declare that they have no competing financial interests.
Podziękowania
The authors thank the National Institute of General Medical Sciences of the National Institutes of Health (NIH) for financial support through an Institutional Development Award (IDeA) under grant number P20GM103541 as well as grant number P20GM10344615. The statements herein do not reflect the views of the NIH. We also acknowledge the Delaware Biotechnology Institute (DBI) and Delaware Economic Development Office (DEDO) for financial support through the Bioscience Center for Advanced Technology (Bioscience CAT) award (12A00448).
Materiały
| Name | Company | Catalog Number | Comments |
| siRNA | Sigma-Aldrich | SIC001 | non-targeted, universal negative control |
| mPEG-b-P(APNBMA) | synthesized in our lab | N/A | photo-responsive polymer |
| HEPES | Fisher Scientific | BP310-100 | |
| sodium dodecyl sulfate | Sigma-Aldrich | 436143 | |
| rubber gasket | McMaster-Carr | 3788T21 | 0.5 mL thick |
| UV laser | Excelitas Technologies | Omnicure S2000 | collimating lens and 365 nm filter used |
| agarose | Fisher Scientific | BP160-100 | |
| ethidium bromide | Fisher Scientific | BP1302-10 | |
| siRNA labelled with Dy547 | GE Healthcare Dharmacon, Inc. | custom order | fluorophore conjugated to 5’ end of sense strand |
| microscope slide | Fisher Scientific | 12-550-A3 | pre-cleaned glass |
| Secure-Seal Spacer | Life Technologies | S24735 | double-sided adhesive |
| LSM 780 | Carl Zeiss | N/A | confocal microscope |
| ZEN 2010 | Carl Zeiss | N/A | FCS analysis software |
| MATLAB | MathWorks | N/A | programming language |
| NIH/3T3 cells | ATCC | ATCC CRL-1658 | |
| DMEM | Mediatech | 10-013-CV | growth media |
| fetal bovine serum | Mediatech | 35-011-CV | heat-inactivated |
| penicillin-streptomycin | Mediatech | 30-002-CI | |
| 6-well plates | Fisher Scientific | 08-772-1B | |
| Opti-MEM | Life Technologies | 11058021 | transfection media |
Odniesienia
- Forbes, D. C., Peppas, N. A. Oral delivery of small RNA and DNA. J Control Release. 162 (2), 438-445 (2012).
- Davis, M. E., et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 464 (7291), 1067-1070 (2010).
- Bouchie, A. Companies in footrace to deliver RNAi. Nat Biotechnol. 30 (12), 1154-1157 (2012).
- Burke, P. A., Pun, S. H., Reineke, T. M. Advancing Polymeric Delivery Systems Amidst a Nucleic Acid Therapy Renaissance. ACS Macro Lett. 2 (10), 928-934 (2013).
- Gooding, M., Browne, L. P., Quinteiro, F. M., Selwood, D. L. siRNA Delivery: From Lipids to Cell-penetrating Peptides and Their Mimics. Chem Biol Drug Des. 80 (6), 787-809 (2012).
- Motta-Mena, L. B., et al. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat Chem Biol. 10 (3), 196-202 (2014).
- Wang, X., Chen, X., Yang, Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods. 9 (3), 266-269 (2012).
- Ziauddin, J., Sabatini, D. M. Microarrays of cells expressing defined cDNAs. Nature. 411 (6833), 107-110 (2001).
- Saltzman, W. M., Olbricht, W. L. Building drug delivery into tissue engineering. Nat Rev Drug Discov. 1 (3), 177-186 (2002).
- Mura, S., Nicolas, J., Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 12 (11), 991-1003 (2013).
- Shim, M. S., Kwon, Y. J. Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications. Adv Drug Delivery Rev. 64 (11), 1046-1058 (2012).
- Kelley, E. G., Albert, J. N. L., Sullivan, M. O., Epps, T. H. Stimuli-responsive copolymer solution and surface assemblies for biomedical applications. Chem Soc Rev. 42 (17), 7057-7071 (2013).
- Weber, W., Fussenegger, M. Emerging biomedical applications of synthetic biology. Nat Rev Genet. 13 (1), 21-35 (2012).
- Konermann, S., et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 500 (7463), 472-476 (2013).
- Shimizu-Sato, S., Huq, E., Tepperman, J. M., Quail, P. H. A light-switchable gene promoter system. Nat Biotechnol. 20 (10), 1041-1044 (2002).
- Huschka, R., et al. Gene Silencing by Gold Nanoshell-Mediated Delivery and Laser-Triggered Release of Antisense Oligonucleotide and siRNA. ACS Nano. 6 (9), 7681-7691 (2012).
- Li, H. -. J., Wang, H. -. X., Sun, C. -. Y., Du, J. -. Z., Wang, J. Shell-detachable nanoparticles based on a light-responsive amphiphile for enhanced siRNA delivery. R Soc Chem Adv. 4 (4), 1961-1964 (2014).
- Braun, G. B., et al. Laser-Activated Gene Silencing via Gold Nanoshell-siRNA Conjugates. ACS Nano. 3 (7), 2007-2015 (2009).
- Gilleron, J., et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 31 (7), 638-646 (2013).
- Wittrup, A., et al. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat Biotechnol. 33 (8), 870-876 (2015).
- Roth, C. M. Quantitative measurements and rational materials design for intracellular delivery of oligonucleotides. Biotechnol Prog. 24 (1), 23-28 (2008).
- Mao, S., et al. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjugate Chem. 17 (5), 1209-1218 (2006).
- Raab, R. M., Stephanopoulos, G. Dynamics of gene silencing by RNA interference. Biotechnol Bioeng. 88 (1), 121-132 (2004).
- Cuccato, G., et al. Modeling RNA interference in mammalian cells. BMC Systems Biology. 5, 1 (2011).
- Varga, C. M., Hong, K., Lauffenburger, D. A. Quantitative analysis of synthetic gene delivery vector design properties. Mol Ther. 4 (5), 438-446 (2001).
- Chen, H. H., et al. Quantitative comparison of intracellular unpacking kinetics of polyplexes by a model constructed from quantum Dot-FRET. Mol Ther. 16 (2), 324-332 (2008).
- Bartlett, D. W., Davis, M. E. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 34 (1), 322-333 (2006).
- Foster, A. A., Greco, C. T., Green, M. D., Epps, T. H., Sullivan, M. O. Light-Mediated Activation of siRNA Release in Diblock Copolymer Assemblies for Controlled Gene Silencing. Adv Healthc Mater. 4 (5), 760-770 (2015).
- Greco, C. T., Epps, T. H., Sullivan, M. O. Mechanistic Design of Polymer Nanocarriers to Spatiotemporally Control Gene Silencing. ACS Biomater Sci Eng. 2 (9), 1582-1594 (2016).
- Green, M. D., et al. Catch and release: photocleavable cationic diblock copolymers as a potential platform for nucleic acid delivery. Polym Chem. 5 (19), 5535-5541 (2014).
- Lee, P. Y., Costumbrado, J., Hsu, C. Y., Kim, Y. H. Agarose Gel Electrophoresis for the Separation of DNA Fragments. J Vis Exp. (62), e3923 (2012).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9 (7), 671-675 (2012).
- Marquer, C., Leveque-Fort, S., Potier, M. C. Determination of Lipid Raft Partitioning of Fluorescently-tagged Probes in Living Cells by Fluorescence Correlation Spectroscopy (FCS). J Vis Exp. (62), (2012).
- Staaf, E., Bagawath-Singh, S., Johansson, S. Molecular Diffusion in Plasma Membranes of Primary Lymphocytes Measured by Fluorescence Correlation Spectroscopy. J Vis Exp. (120), e54756 (2017).
- Buyens, K., et al. Monitoring the disassembly of siRNA polyplexes in serum is crucial for predicting their biological efficacy. J Control Release. 141 (1), 38-41 (2010).
- Eden, E., et al. Proteome Half-Life Dynamics in Living Human Cells. Science. 331 (6018), 764-768 (2011).
- Towbin, H., Staehelin, T., Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad. Sci U S A. 76 (9), 4350-4354 (1979).
- Wittwer, C. T., Herrmann, M. G., Moss, A. A., Rasmussen, R. P. Continuous Fluorescence Monitoring of Rapid Cycle DNA Amplification. Biotechniques. 54 (6), 314-320 (2013).
- Cho, S. K., Dang, C., Wang, X., Ragan, R., Kwon, Y. J. Mixing-sequence-dependent nucleic acid complexation and gene transfer efficiency by polyethylenimine. Biomater Sci. 3 (7), 1124-1133 (2015).
- Liu, Y. M., Reineke, T. M. Poly(glycoamidoamine)s for gene delivery: Stability of polyplexes and efficacy with cardiomyoblast cells. Bioconjugate Chem. 17 (1), 101-108 (2006).
- Schwanhausser, B., et al. Global quantification of mammalian gene expression control. Nature. 473 (7347), 337-342 (2011).
- Greco, C. T., Muir, V. G., Epps, T. H., Sullivan, M. O. Efficient tuning of siRNA dose response by combining mixed polymer nanocarriers with simple kinetic modeling. Acta Biomater. 50, 407-416 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone