Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Evaluating In Vitro DNA Damage Using Comet Assay
W tym Artykule
Podsumowanie
The comet assay is an efficient method to detect DNA damage including single and double-stranded DNA breaks. We describe alkaline and neutral comet assays to measure DNA damage in cancer cells to evaluate the therapeutic effect of chemotherapy.
Streszczenie
DNA damage is a common phenomenon for each cell during its lifespan, and is defined as an alteration of the chemical structure of genomic DNA. Cancer therapies, such as radio- and chemotherapy, introduce enormous amount of additional DNA damage, leading to cell cycle arrest and apoptosis to limit cancer progression. Quantitative assessment of DNA damage during experimental cancer therapy is a key step to justify the effectiveness of a genotoxic agent. In this study, we focus on a single cell electrophoresis assay, also known as the comet assay, which can quantify single and double-strand DNA breaks in vitro. The comet assay is a DNA damage quantification method that is efficient and easy to perform, and has low time/budget demands and high reproducibility. Here, we highlight the utility of the comet assay for a preclinical study by evaluating the genotoxic effect of olaparib/temozolomide combination therapy to U251 glioma cells.
Wprowadzenie
The comet assay was first developed by Ostling and Johanson in 1984 by demonstrating the migration of DNA fragments from nuclei under a neutral condition1. The technique was later developed by Singh et al., showing that an alkaline condition substantially increased the specificity and reproducibility of the assay2. Since then, the neutral comet assay is mostly used to detect double-stranded DNA breaks, whereas the alkaline comet assay is more sensitive for smaller amounts of DNA damage, including single and double strand DNA breaks, alkali-labile sites, DNA-DNA or DNA-protein cross-linking, and DNA single-strand breaks associated with incomplete excision repair sites3,4. Both assays allow visualization of fragmented DNA, and provide a straightforward way to quantitatively evaluate DNA damage. The comet assay is regarded as a sensitive method for in vitro and in vivo genetic toxicological studies and is applicable to different research areas, such as early drug-candidate selection, environmental monitoring, human biomonitoring, and fundamental research in DNA damage and repair5.
The principle of the assay is that under an electric field, fragmented DNA migrates out of the nucleoid body (also known as the "comet head") and forms a DNA stain in the agarose gel (also known as the "comet tail"). With nucleotide staining, the extent of DNA damage can be quantified by analyzing "comets" formed by this single cell electrophoresis. Calculation of the tail moment can further help to compare DNA damage among different experimental groups. Compared with traditional methods of DNA damage detection, the comet assay is direct, sensitive, inexpensive, and relatively simple.
Radiotherapy and chemotherapies are common strategies for cancer treatment by generating single strand and double strand DNA breaks in chromosomes6. The recent advancement in DNA repair inhibitors allows a more effective genotoxic effect by combination chemotherapy, and therefore, potentially reduces systemic side effects such as anemia, infection, and bone marrow suppression7,8. In this study, we showed the investigation of a poly (ADP-ribose) polymerase (PARP) inhibitor, olaparib (Ola)9. PARP is an abundant nuclear protein and is responsible for DNA base excision repair by forming a poly (ADP-ribose) polymer10. Temozolomide (TMZ) is an orally available alkylating agent and has been widely used for glioma patient treatment. By using the comet assay to quantify DNA damage, we demonstrate that combining olaparib with temozolomide profoundly enhances the DNA damage in glioma cells, which suggests olaparib/temozolomide combination therapy is an effective strategy to treat glioma, as compared with temozolomide alone11.
Protokół
1. Prepare Reagents
- 1x PBS
- Dilute 100 mL 10x PBS with 900 mL dH2O and adjust the pH to 7.4 using a pH meter. Store at room temperature.
- Lysis solution (LS)
- Prepare 2.5 M NaCl, 100 mM disodium EDTA, 10 mM Tris base, and 200 mM NaOH in 900 mL dH2O; it commonly takes about 20 min to allow the mixture to fully dissolve. Adjust the pH to 10 using a pH meter. Add 1% sodium lauryl sarcosinate and 1% Triton X-100, and adjust the final volume to 1,000 mL. Cool to 4 °C for at least 30 min before use.
- Alkaline electrophoresis solution (AES), pH >13
- Prepare 200 mM NaOH and 1 mM disodium EDTA in 800 mL dH2O. Adjust the pH and make sure that it is pH >13. Adjust the final volume to 1,000 mL. Make fresh before use and cool to 4 °C for at least 30 min before use.
- Neutral electrophoresis sollution (NES)
- Prepare 1,000 mL neutral electrophoresis buffer by mixing 100 mM Tris base and 300 mM sodium acetate to 1,000 mL dH2O. Adjust the pH to 9.0 with glacial acetic acid. Cool to 4 °C for at least 30 min before use.
- DNA precipitation solution (DPS)
- Preparation of 10 mL 7.5 M ammonium acetate stock. For 50 mL of DNA precipitation solution, mix 6.7 mL 7.5 M ammonium acetate with 43.3 mL 95% ethanol. Store at room temperature.
- Staining solution
- Add 1 µL 10,000x green fluorescent nucleic acid stain (e.g., SYBR Green) in 30 mL Tris-EDTA buffer (10 mM Tris-HCl, 1 mM disodium EDTA, pH 7.4) and store at 4 °C. Protect from light.
- 1% low melting agarose
- Melt 1% low melting point agarose (1 g in 100 mL dH2O) in a microwave. Swirl the agarose every 15-20 s to make sure that the agarose is completely molten. Place the agarose in 37 °C water bath for at least 20 min before use.
- Pre-warm pipette tips
- Cut off the narrow ends of P200 pipette tips by 3 mm and warm at 37 °C before pipetting agarose.
2. Prepare Comet Slides
- Slide coating
- Melt 1% agarose (1 g in 100 mL dH2O) in a microwave for 2 - 3 min or until the agarose is completely molten. Dip the glass microscope slides into the agarose and wipe one side of the slide using a lint-free wipe.
- Lay the slides on a flat surface to air-dry or heat at 50 °C for faster drying; a transparent agarose film should be formed after drying. Place the coated slides in 37 °C before use.
- Preparation of single cell suspensions
- Culture and treat the glioma cell
- Culture the U251 MG cells in DMEM-Ham F-12 medium supplemented with 10% FBS, 100 U/mL penicillin, and 10 µg/mL streptomycin at 37 °C with 5% CO2.
- Digest the cells using 1 mL trypsin for 3 min, and neutralize trypsin using DMEM-Ham F-12 medium with FBS. Collect in 15 mL tube, spin at 300 x g for 4 min, aspirate the medium, and suspend cells at 2 x 105 cell/mL in 1x PBS.
NOTE: The cell sample should be prepared immediately before starting the assay and all samples should be handled in a dark or dimmed environment to prevent DNA damage from light. - Combine the cell suspension with 1% molten low melting point agarose (at 37 °C) at a ratio 1:10 (v/v), mix gently by pipetting up and down, and immediately pipette 30 µL onto a slide. Use the side of the the pipette tip to spread the agarose/cell mixture to ensure the formation of a thin layer.
- Place the slide flat at 4 °C in the dark for 10 min. Increasing the gelling time to 30 min improves adherence of samples in high humidity environments.
- Immerse the slide in 4 °C LS in the dark for 1 h to overnight.
- Culture and treat the glioma cell
3. Single Cell Electrophoresis
- Proceed to alkaline (step 3.2) or neutral (step 3.3) comet assay
- For alkaline comet assay
- Gently remove slides from the LS, drain excess buffer, and gently immerse in AES for 1 h at 4 °C to allow DNA unwinding. Keep the slides in the dark.
- Add pre-chilled AES in the electrophoresis slide tray, do not exceed 0.5 cm above the slides (this depends on the size of the electrophoresis units), place the slides inside and cover with a cap. Set the power supply voltage to 1 V/cm (the length between electrodes) and run for 30 min at 4 °C.
- Drain excess electrophoresis solution from slide. Gently immerse slides twice in dH2O for 5 min each at room temperature.
- Gently immerse slides in 70% ethanol for 5 min at room temperature. Proceed to step 4.
- For neutral comet assay
- Gently remove the slides from the LS, drain excess buffer, and gently immerse in NES for 30 min at 4 °C. Keep the slide in the dark.
- Add pre-chilled neutral electrophoresis buffer in the electrophoresis slide tray, do not exceed 0.5 cm above slides (this depends on the size of the electrophoresis units), place the slides inside and cover with a cap. Set the power supply voltage to 1 V/cm (the length between electrodes) and run for 45 min at 4 °C.
- Drain excess buffer from the slides. Gently immerse slides in DPS for 30 min at room temperature.
- Gently immerse the slides in 70% ethanol for 30 min at room temperature. Proceed to step 4.
4. Stain Comet Slides
- Dry slides at 37 °C for 10 - 15 min in the dark.
- Place 50 - 100 µL green fluorescent nucleic acid staining solution onto each dried agarose and stain for 15 min at room temperature in the dark.
- Rinse the slides briefly in dH2O and dry completely at 37 °C in the dark. Proceed to image acquisition and analysis.
5. Image Acquisition and Analysis
NOTE: The visualization and quantification of DNA breaks are based on epifluorescence microscopy and the comet assay software (see Table of Materials)12.
- Place the slides on the microscope with a slide holder. Ensure the agarose gel is facing to the objective lens. Randomly capture images from the stained comet slides using a fluorescence microscope with a 10x objective lens. Avoid the edges and the areas around any air bubbles.
- Ensure each comet tail is horizontally distributed. Comet heads should originate from the left and the tail from the right.
- Save each picture in a binary TIF format with bright DNA stain and dark background. Load images to the software using the "Select files to analyze" button, which is located on the left of the toolbar. An image view window should appear (Figure 1).
- Draw a measurement frame on the screen and adjust its size in accordance with the comet of the cell. Click the "Adjust" button to set up the threshold of the head, comet, and tail according to the image, then click the "Start measurements" button (Figure 1).
- Select a cell using the frame and activate the measurement by clicking with the mouse on the "Assay the comet" button; an intensity image shows up on the "profiles" window with the selected measurement parameters. The results can be saved by clicking the "store result" button (Figure 1).
NOTE: The software calculates the parameters including the length of the comet tail, the percentage of the tailed DNA, the tail moment (TM) and the Olive tail moment (OTM). The tail moments are calculated by the formulas as follow:

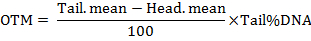
- Analyze at least 50 cells per treatment.
Wyniki
The present protocol describes a step-by-step workflow for the comet assay execution and data analysis (Figure 1). Results from the alkaline and neutral comet assays showed that the comet tail of doxorubicin-treated U251 cells (1 µM, 20 h) was longer and had higher DNA intensity, suggesting a substantial accumulation of fragmented DNA due to chemotherapy (Figure 2).
Qu...
Dyskusje
The comet assay is an efficient tool to measure single and double-strand DNA breaks at the cellular level. The assay has been widely applied as a "golden standard" in studies regarding genotoxicity and biomonitoring13, ranging from base lesions, DNA crosslinks, drug development, and alkali sensitive sites. In the present study, we showed two distinct step-by-step protocols for alkaline and neutral comet assays, respectively. Combining single cell electrophoresis, fluorescent microscopy, and image ...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This research was supported by the Intramural Research Program of the NIH, NCI, and CCR. All authors received Intramural Research Grant from NIH, NCI, and CCR.
Materiały
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| 10x PBS(Ca++, Mg++ free) | TEKnova | P0196 | |
| NaCl | Sigma | S5886 | |
| EDTA | TEKnova | E0308 | |
| Trizma base | Sigma | T1503 | |
| NaOH | Sigma | 72068 | |
| Sodium lauryl sarcosinate | Sigma | L7414 | |
| Triton X-100 | Sigma | 93443 | |
| Sodium acetate | Sigma | 32318 | |
| Glacial acetic acid | Sigma | 695092 | |
| Ammonium acetate | Sigma | A1542 | |
| SYBR Green | Invitrogen | S33102 | |
| Low melting point agarose | Invitrogen | 16520 | |
| Agarose | Invitrogen | 16500 | |
| 95% ethanol | WARNER-GRAHAM | #64-17-5 | |
| Trypsin | GIBICO | 25300-054 | |
| Name | Company | Catalog Number | Comments |
| Consumables | |||
| Glass tissue slides | ELECTRON MICROSCOPY SCIENCES | 63422-11 | |
| Kimwipes | KIMberly-Clark | ||
| 1.5 mL Microcentrifuge Tubes | DENVILLE | ||
| Pipette Tips | SHARP | ||
| Name | Company | Catalog Number | Comments |
| Equipments | |||
| Microwave | Avanti | ||
| Waterbath | PRECISION | ||
| Horizontal electrophoresis chamber | TREVIGEN | Cometassay ES II | |
| Power supply | Bio-Rad | ||
| Incubator | Quincy Lab | Model 12-140E | |
| Fluorescent microscope | Zeiss | LSM700 | |
| Micropipettor | Eppendorf |
Odniesienia
- Ostling, O., Johanson, K. J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 123 (1), 291-298 (1984).
- Singh, N. P., McCoy, M. T., Tice, R. R., Schneider, E. L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 175 (1), 184-191 (1988).
- Tice, R. R., et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 35 (3), 206-221 (2000).
- Shah, A. J., Lakkad, B. C., Rao, M. V. Genotoxicity in lead treated human lymphocytes evaluated by micronucleus and comet assays. Indian J Exp Biol. 54 (8), 502-508 (2016).
- Azqueta, A., Collins, A. R. The essential comet assay: a comprehensive guide to measuring DNA damage and repair. Arch Toxicol. 87 (6), 949-968 (2013).
- Goldstein, M., Kastan, M. B. The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med. 66, 129-143 (2015).
- Gavande, N. S., et al. DNA repair targeted therapy: The past or future of cancer treatment?. Pharmacol Ther. 160, 65-83 (2016).
- Torgovnick, A., Schumacher, B. DNA repair mechanisms in cancer development and therapy. Front Genet. 6, 157 (2015).
- Weston, V. J., et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood. 116 (22), 4578-4587 (2010).
- Brown, J. S., O'Carrigan, B., Jackson, S. P., Yap, T. A. Targeting DNA Repair in Cancer: Beyond PARP Inhibitors. Cancer Discov. 7 (1), 20-37 (2017).
- Lu, Y., et al. Chemosensitivity of IDH1-Mutated Gliomas Due to an Impairment in PARP1-Mediated DNA Repair. Cancer Res. 77 (7), 1709-1718 (2017).
- Konca, K., et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res. 534 (1-2), 15-20 (2003).
- Valverde, M., Rojas, E. Environmental and occupational biomonitoring using the Comet assay. Mutat Res. 681 (1), 93-109 (2009).
- Collins, A. R. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 26 (3), 249-261 (2004).
- Karbaschi, M., Cooke, M. S. Novel method for the high-throughput processing of slides for the comet assay. Sci Rep. 4, 7200 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone