Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
In Vivo EPR Assessment of pH, pO2, Redox Status, and Concentrations of Phosphate and Glutathione in the Tumor Microenvironment
W tym Artykule
Podsumowanie
Low-field (L-band, 1.2 GHz) electron paramagnetic resonance using soluble nitroxyl and trityl probes is demonstrated for assessment of physiologically important parameters in the tumor microenvironment in mouse models of breast cancer.
Streszczenie
This protocol demonstrates the capability of low-field electron paramagnetic resonance (EPR)-based techniques in combination with functional paramagnetic probes to provide quantitative information on the chemical tumor microenvironment (TME), including pO2, pH, redox status, concentrations of interstitial inorganic phosphate (Pi), and intracellular glutathione (GSH). In particular, an application of a recently developed soluble multifunctional trityl probe provides unsurpassed opportunity for in vivo concurrent measurements of pH, pO2 and Pi in Extracellular space (HOPE probe). The measurements of three parameters using a single probe allow for their correlation analyses independent of probe distribution and time of the measurements.
Wprowadzenie
A key role of the TME in cancer progression and therapy is increasingly appreciated1. Among important physiological parameters of the TME in solid tumors, tissue hypoxia2, acidosis3,4, high reducing capacity5, elevated concentrations of intracellular GSH6,7, and interstitial Pi8 are well documented. Noninvasive in vivo pO2, pH, Pi, GSH, and redox assessments provide unique insights into the biological processes in TME, and help advance tools for pre-clinical screening of anti-cancer drugs and TME-targeted therapeutic strategies. A reasonable radiofrequency penetration depth in tissues by magnetic resonance imaging (MRI) and low-field EPR-based techniques makes them the most appropriate approaches for noninvasive assessment of these TME parameters. MRI relies largely on imaging water protons and is widely used in clinical settings to provide anatomical resolution but lacks functional resolution. The phosphorus-31 nuclear magnetic resonance (31P-NMR) measurements of extracellular Pi concentration and pH based on a signal from endogenous phosphate are potentially attractive for TME characterization, but are normally masked by several times higher intracellular Pi concentrations9,10. In contrast to this, EPR measurements rely on spectroscopy and imaging of specially designed paramagnetic probes to provide functional resolution. Note that exogenous EPR probes have an advantage over exogenous NMR probes due to the much higher intrinsic sensitivity of EPR and absence of endogenous background EPR signals. The recent development of a dual function pH and redox nitroxyl probe11 and multifunctional trityl probe12 provides unsurpassed opportunities for in vivo concurrent measurements of several TME parameters and their correlation analyses independent on probe distribution and time of measurement. To our knowledge, there are no other methods available to concurrently assess in vivo physiologically important chemical TME parameters in living subjects, such as pO2, pHe, Pi, redox, and GSH.
Probes for In Vivo Functional Measurements:
Figure 1 shows chemical structures of the paramagnetic probes used to access TME parameters, which include particulate and soluble probes. High functional sensitivity, stability in living tissue, and minimal toxicity are a few benefits that make particulate probes preferred over soluble probes for in vivo EPR oximetry. For example, particulate probes have increased retention times at the site of tissue implant compared to soluble probes allowing for longitudinal measurement of tissue pO2 over several weeks. On the other hand, soluble probes outperform particulate probes by providing spatial-resolved measurements using EPR-based imaging techniques as well as allowing concomitant analyses from multiple functionalities (pO2, pH, Pi, redox, and GSH).

Figure 1. Chemical structures of the paramagnetic probes that assemble TME assessment assay. This includes the particulate pO2 probe, LiNc-BuO (R = -O(CH2)3CH3), and soluble probes: dual function pH and redox probe, NR; GSH-sensitive probe, RSSR; and multifunctional pO2, pH, and Pi probe of the extracellular microenvironment, the HOPE probe. The synthesis of these probes has been described in the provided references 11,12. Please click here to view a larger version of this figure.
Protokół
All animal work was performed in accordance with WVU IACUC approved protocol.
1. Probe Synthesis and Calibration
- Particulate pO2-sensitive LiNc-BuO probe
Note: LiNc-BuO microcrystals are synthesized and prepared as described in reference13. They are very stable and can be kept at room temperature for years. The EPR linewidth of the LiNc-BuO particulate probe is a pO2-sensitive parameter. LiNc-BuO microcrystals demonstrate ideal linear dependence of the linewidth on oxygen concentration in the range from anoxic conditions up to 760 mmHg of pO2 partial pressure13, with the values of intrinsic linewidth in the absence of oxygen and the slope of the oxygen dependence (measured in mG/mmHg) slightly varying for different batches of the microcrystal preparation. Therefore, calibration is required for every particular batch.- Weigh 60 mg of LiNc-BuO microcrystals.
- For oxygen sensitivity calibration, suspend microcrystals in 3 mL of Dulbecco's Modified Eagle Media (DMEM) medium (in a concentration of 20 mg/mL) and sonicate for 5 min on ice with a probe sonicator at 20 kHz using 7 W power in a 5-mL glass round-bottom tube.
- Place 1 mL of the sonicated microcrystals in a glass tube in the surface coil resonator of the L-band (1.2 GHz) EPR spectrometer and acquire continuous waves (CW) EPR spectra at the physiological temperature of 37 °C and oxygen concentrations of 0, 1, 2, 4, 8, and 20.9%. Maintain the oxygen concentration by bubbling the solution with gas mixture delivered from a gas controller and maintain the temperature using a water bath attached to a thermostat. Use the following EPR spectrometer acquisition parameters: modulation amplitude, 100 mG; modulation frequency, 100 kHz; sweep width, 5 G; sweep time, 60 s.
- To achieve a better signal-to-noise ratio (SNR), use a modulation amplitude value of 60% of the linewidth (e.g., use a modulation amplitude 0.6 G for the linewidth of 1 G).
- Alternative simplified calibration procedure: Record the EPR spectra in air-bubbled and anoxic solutions14. In the latter case, maintain anoxia in the samples by the addition of 10 mM glucose and 100 U/mL glucose oxidase to 1 mL probe solutions according to reference14.
- Fit the EPR spectra with the Lorentzian function to find the line width, LW. Evaluate the sensitivity of microcrystals to pO2 as a slope of the dependence of LW on pO2, namely as a value of (LWair−LWanoxia)/pO2air, where LWair and LWanoxia are spectra linewidth in air and anoxia conditions, respectively; pO2air = 152 mmHg.
- Dual function pH and redox probe, NR
Note: The NR probe is synthesized as described in reference11. It is stable at room temperature both as a solid and in aqueous solutions. The synthesized NR probe is kept at 4 °C. The nitrogen hyperfine splitting, aN, and the signal amplitude decay rate are the spectral parameters of the NR probe that are sensitive to pH (probe pKa = 6.6 at 37 °C, range of pH sensitivity from 5.6 to 7.6) and to the reducing capacity of the probe microenvironment, respectively.- Remove the NR from the freezer and allow the container to warm to room temperature (10 - 15 min). Weigh out 6.34 mg of the NR, dissolve it in 1 mL of saline solution, and adjust the pH to 7.2 with small aliquots of HCl or NaOH using a pH meter. Use the prepared NR solution (10 mM) as a stock solution.
- Perform the pH calibration of the NR probe as follows (see reference11). First, add 0.1 mL of the NR stock solution to 0.9 mL of 2 mM Na-phosphate buffer, 150 mM NaCl. Titrate the obtained 1 mM NR solution with aliquots of HCl or NaOH to the required pH using a pH meter. Control the temperature using a water bath attached to a thermostat.
- Record the EPR spectra of the samples in 1.5-mL microcentrifuge tubes using the L-band EPR spectrometer. Use the following EPR spectrometer acquisition parameters: modulation amplitude, 2.5 G; modulation frequency, 100 kHz; sweep width, 60 G; sweep time, 20 s.
- Measure the hyperfine splitting constant (aN) as half of the distance between low- and high-field components of the EPR spectra and plot it versus pH, to provide the calibration curve for L-band EPR measurements of pH.
- GSH-sensitive RSSR probe
Note: The RSSR probe is synthesized as described in reference15. Store the synthesized NR probe at 4 °C. The lipophilic RSSR disulfide biradical compound easily diffuses across the cell membrane to react with intracellular GSH and provide a reliable approach for determining GSH in vivo using EPR16,17. This method is based on the high reaction rates of the predominant intracellular pool of GSH thiols with the RSSR probe. The reaction of the RSSR biradical with GSH splits its disulfide bond (see Scheme 1) resulting in the cancellation of spin exchange between the two radical fragments and manifesting in a decrease of the biradical spectral components and corresponding increase of the monoradical components. For the biradical RSSR probe, the rate of the increase of the amplitude of the monoradical component is proportional to the GSH concentration and is a convenient GSH-sensitive EPR spectral parameter. To evaluate GSH concentration from the in vivo EPR measurements, the preceding calibration of the rate of the RSSR reaction with GSH at the corresponding temperature and pH has to be performed as follows.- Remove the RSSR from the freezer and allow the container to warm to room temperature (10-15 min). Weigh out 4.05 mg of the NR and dissolve it in 1 mL of DMSO solution. Use the prepared RSSR solution (10 mM) as a stock solution.
- Determine the value of the rate constant, kobs, of the reaction of RSSR with GSH at the desirable temperature and pH as follows.
- First, add 20 µL of the RSSR stock solution (10 mM) to 0.98 mL of 1 mM Na-phosphate buffer, pH 7.2, 150 mM NaCl, to obtain a 0.2 mM RSSR probe solution.
- Prepare solutions of 1, 2, and 5 mM concentrations of GSH in 0.1 M Na-phosphate buffer at pH 7.2. To accurately evaluate GSH concentration in the cells of the targeted organ from the in vivo measurements, the in vitro calibration has to be performed at a pH close to the value of the intracellular pH.
- Mix equal volumes of 0.2 mM RSSR solution and one of the GSH solutions prepared in step 1.3.4. for a final concentration of the probe at 0.1 mM and of GSH at 0.5, 1, or 2.5 mM.
- Immediately after RSSR and GSH solution mixing, place the sample in the EPR resonator and record the EPR spectra every 12 seconds for 10 minutes. Then calculate the kinetics of the increase of the monoradical spectral amplitude. Use the following EPR spectrometer acquisition parameters: modulation amplitude, 1 G; modulation frequency, 100 kHz; sweep width, 60 G; sweep time, 10-60 s.
- Fit the measured EPR kinetics to the monoexponents and calculate the time constant of the exponential kinetics, τ. The linear regression (1/τ = kobs × [GSH]) provides the observed rate constant value of the reaction between GSH and RSSR (e.g., at 34 °C and pH 7.2, kobs = 2.8 ± 0.2 M-1s-1)11.
- Multifunctional HOPE probe for pO2, pH, and Pi assessment
Note: The monophosphonated trityl HOPE probe is synthesized as described in reference12 and is kept at 4 °C. The CW EPR spectra of HOPE at pH << pKa (A - acid form) and pH >> pKa (B - basic form) are represented by the doublets due to phosphorus hyperfine splitting, aP. The typical instrument settings are as follows: modulation amplitude, 37.5 mG; modulation frequency, 100 kHz; sweep width, 0.9 G; sweep time, 20-60 s. At intermediate pH (5 < pH < 8) the EPR spectrum of HOPE probe is characterized by a quartet when both A and B states are present. The individual EPR linewidth of the HOPE is a pO2 marker (accuracy, ≈ 1 mmHg; pO2 range, 1-100 mmHg). The fraction of protonated HOPE (A form) is a pH marker in the range from 6 to 8.0 (accuracy, ± 0.05). The value of the proton exchange rate (expressed in mG) with Pi extracted by spectra simulation is a Pi marker (accuracy, ± 0.1 mM, range, 0.1-20 mM). Calibration procedures are performed at physiological temperature (37 °C), solution ionic strength (NaCl, 150 mM), and HOPE probe concentration of 0.2 mM, as previously described in references12,18, and detailed below.- Remove the HOPE probe from the freezer and allow the container to warm to room temperature (10-15 min).
- Weigh out 10.7 mg of the HOPE probe, dissolve it in 1 mL of saline solution, and adjust the pH to 7.4. Add 20 µL of the prepared stock solution of the HOPE (10 mM) to 0.98 mL of the saline solution to obtain a 0.2 mM HOPE probe solution.
- For probe calibration of the pH, titrate 0.2 mM of the HOPE probe solution by the addition of a small volume of NaOH or HCl, with the final dilution of sample less than 1%. Measure the pH with a pH electrode calibrated at 37 °C using the pH values for the reference solution recommended by National Bureau of Standards (U.S.). Use a jacketed reaction beaker attached to a circulator to carefully maintain temperature of the reference and titrated solutions during pH measurements. Maintain anoxic conditions by the addition of 10 mM glucose and 100 U/mL glucose oxidase to the probe solutions.
- Acquire the EPR spectra of the A and B forms at pH ≤ 5 and pH ≥ 8, respectively, in anoxic conditions in the absence of phosphate.
- Use the corresponding spectra to obtain intrinsic spectral parameters. Namely, simulate the spectral line as the convolution of the Lorentzian function with the Gaussian function that approximates the unresolved super hyperfine structure of the HOPE probe. The fitting of the calculated EPR spectra to the experimental spectra yields the values of aP and Lorentzian linewidth (ΔLpp), determined by the transverse relaxation rate, 1/T2 (where 1/T2 = (√3/2)Lpp for the measured derivative of the RF absorption line in CW EPR), and the linewidth of Gaussian distribution, G.
Note: The following are the parameters obtained from the spectra measured at the conditions specified in step 1.4.2: aP(A) = 3.63 G, aP(B) = 3.37 G; 1/T2(A) = 23.6 mG; 1/T2(B) = 9 mG; G(A) = 40 mG; G(B) = 45 mG (see reference8). - Acquire the EPR spectra of the HOPE at intermediate pH (5 < pH < 8). Simulate the high-filed component of the acquired EPR spectra using the theory of exchange between several sites in non-coupled or loosely coupled systems adapted from reference19 as previously described18. Use the intrinsic parameters obtained for A and B states (see step 1.4.5) to decrease the number of variables. Fit the calculated spectra to the experimental ones to find the values of the fraction A (pA), and plot the dependence of the pA value on pH. Use the dependence of pA on pH in further studies as a pH calibration curve.
Note: Fitting the pH dependence of pA with a standard titration curve provides the value of the dissociation constant, pKa (HOPE) = 6.98. In in vivo studies, acquiring the full EPR spectra of the HOPE probe is impractical because of the additional time needed to acquire the gap between low- and high-field components of the phosphorus hyperfine splitting in the EPR spectrum. Therefore, in further exemplified studies only the high-filed component of the EPR spectrum has been measured and analyzed. - For probe calibration of pO2, acquire EPR spectra of the HOPE probe at various oxygen concentrations.
- Control the pO2 values of the solutions by bubbling with gas mixture delivered from a gas controller. Control the solution temperature (37 °C) using a water bath attached to a thermostat.
- Simulate the EPR spectra and fit them to the experimental ones as described in step 1.4.6 to determine the values of oxygen-induced relaxation rates.
Note: The values of oxygen-induced relaxation rates were 0.49 mG/mmHg both for A and B forms of the HOPE probe as measured at 37 °C8. - For probe calibration of [Pi], acquire EPR spectra of the HOPE probe at various phosphate concentrations. Use the HOPE radical solution with a pH value near pKa (pKa = 6.9 at 37 °C)18 and titrate it with various phosphate concentrations. Maintain the temperature and gas composition as described in above steps.
- Simulate the EPR spectra and fit them to the experimental ones as described in step 1.4.6 to determine the values of Pi-induced exchange rate.
Note: The dependence of the Pi-induced exchange rate on [Pi] is used as calibration in further studies.
2. Mouse Models of Breast Cancer
- MMTV-PyMT spontaneous tumor model
- Use 4-8 week-old female Friend virus B-type susceptibility/NIH (FVB/N) mouse mammary tumor virus promoter (MMTV) polyoma middle-T antigen (PyMT+) mice with spontaneously formed mammary tumors for in vivo EPR studies.
- For comparison of tissue microenvironments of normal mammary glands and tumors, use age-matched littermate females deficient in the PyMT oncogene (PyMT−, "wild type")20.
- Subject the mice to L-band EPR spectroscopy once per week for four weeks during isoflurane anesthesia (see probe delivery below).
- Anesthetize the mouse using an air-isoflurane mixture (3% isoflurane), and place the mouse on an adjustable table in a right, lateral position with the tumor (mammary glands) close to the surface coil resonator.
- After mouse placement, administer the probe by intratissual (i.t.) injection, tune the EPR spectrometer, and acquire the EPR spectra for 5-10 min.
- Measure 2-3 mammary tumors (from MMTV-PyMT+ mice) or non-tumor bearing mammary glands (from PyMT− mice) during the same EPR session.
- Orthotopic MET-1 tumor model
- Grow FVB/N background Met-1 murine breast cancer cells at 37 °C, 5% CO2, and 95% relative humidity in DMEM containing 10% fetal bovine serum (FBS), 10 µg/mL insulin, 5 ng/mL rhEGF, and 1% PSA (penicillin G sodium, streptomycin sulfate, and amphotericin B) to ~ 80% confluence in a T175 flask.
- Aspirate the media and rinse the adherent cells with 10 mL PBS (1.54 mM KH2PO4, 155 mM NaCl, and 2.71 mM Na2HPO4-7H2O without calcium chloride or magnesium chloride, pH = 7.4).
- Detach the cells by adding 5 mL of 0.25% Trypsin-EDTA solution and rocking the flask. When the cells are detached, add 10 mL DMEM containing 10% FBS to the flask and collect the cells.
- Centrifuge the cell suspension at 132 x g for 10 min at 4 °C. Count the cells using a hemocytometer and resuspend to 1 x 106 cells per 100 µL minimal DMEM.
- Using an insulin syringe (29G1/2 needle), slowly inject 100 µL of tumor cell suspension into the number 4 mammary fat pads of 8-week-old female FVB/N wild type mice.
- Monitor tumor initiation by palpation (appear after approximately 2-3 weeks), growth (visual), and mouse heath (visual) every other day.
- Measure tumor dimensions once per week using calipers and determine tumor volumes using the equation:
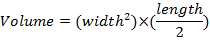
3. Probe Delivery for In Vivo Functional Measurements
- Use particulate LiNc-BuO probe (Protocol I) for pO2 measurements in orthotopic tumor models by implanting tumor cells with internalized LiNc-BuO microcrystals as previously described14,21 and detailed below.
- In the case of the MET-1 tumor model, for internalization of LiNc-BuO microcrystals into MET-1 cells, suspend the LiNc-BuO microcrystals in DMEM at a concentration of 20 mg/mL and sonicate with a probe sonicator at 20 kHz using 7 W power in a 5-mL glass round-bottom tube for 5 min on ice.
- Add 100 µL (2 mg of LiNc-BuO) of the suspension to a T75 flask with 10 mL culture media containing MET-1 cells (approximately 30% confluent). All procedures take place in a biosafety cabinet and the media contains penicillin and streptomycin to minimize potential infection.
- Incubate the cells at 37 °C for 72 h or until they reach ~ 80% confluency.
- Aspirate the media. Wash the cells five times with 10 mL PBS. Detach the cells with 5 mL trypsin-EDTA. Collect the cells. Centrifuge as described above in step 2.2.4. Stain a sample of cells with exclusion dye to determine cell viability and quantity.
- Suspend the cells at a concentration of 1 x 106 per 100 µL minimal DMEM.
- Using an insulin syringe, slowly inject 100 µL of cell suspension containing the internalized LiNc-BuO microcrystals into the number 4 mammary fat pads of 8-week-old female FVB/N wild type mice as described in step 2.2.5.
- Monitor tumor initiation and growth as described in steps 2.2.6 and 2.2.7.
- Use particulate LiNc-BuO probe (Protocol II) in either spontaneous or orthotopic models. Inject LiNc-OBu microcrystals at the site of interest, e.g., into normal mammary glands or mammary tumors, using an insulin syringe.
- Soluble probes
- Anesthetize the mice by inhalation of an air-isoflurane mixture (1.0 L/min delivery and 2-3% of isoflurane) using an anesthesia machine and place them into the gap of the EPR spectrometer.
- Tune the instrument, then inject the NR (10-30 µL, 10 mM), HOPE probe (10−30 µL, 0.5-2 mM) in saline, pH 7.2, or RSSR probe in DMSO (10 µL, 10 mM) solutions (i.t.).
4. In Vivo Functional Measurements
- For EPR spectroscopic measurements, anesthetize the mice by inhalation of air-isoflurane mixture using an anesthesia machine as described in step 3.3.1.
- Perform functional measurements using L-band (1.2 GHz) EPR spectrometer as follows.
- Place the surface coil resonator onto a normal mammary gland or a mammary tumor and tune the spectrometer.
- Acquire the EPR spectra from the implanted particulate probe for 5−10 min over several weeks after implantation. In the case of soluble probes, acquire the EPR spectra immediately after probe injection for 5-10 min.
- Analyze the EPR spectra of the NR probe to find the hyperfine splitting, aN, and signal amplitude, I(t). Convert the value of aN to the pH value using the calibration curve obtained in step 1.2.4. Analyze the rate of decay of the signal amplitude I(t) as relative change from the initial amplitude, I(t = 0), calculated in arbitrary units per second (s-1).
- Fit the increase of the monoradical component of the EPR spectrum of GSH-sensitive RSSR probe to the monoexponents to obtain the time constant of the exponential kinetics to calculate GSH concentration.
- Fit the EPR spectra of high-field component of the multifunctional HOPE probe to the experimental ones as described in (step 1.4.5) to yield the values of pH, pO2 and Pi.
5. Statistical Analysis
- Perform data processing and statistical analyses. Use a Pearson's r Correlation test (for normally distributed datasets) and Spearman's Rank Order Correlation (for datasets with rejected normality of data distribution) for correlation analyses.
Wyniki
Tissue pO2 Assessment Using the LiNc-BuO Probes:
Using the procedure described under step 1.1, we performed the calibration of freshly prepared LiNc-BuO microcrystals suspension. Figure 2 shows the typical oxygen dependence of the linewidth of the LiNc-BuO probe, as well as its exemplified EPR sp...
Dyskusje
The presented methods allow for noninvasive in vivo assessment of the critical parameters of the chemical TME, namely pO2, pH, redox status, and concentrations of interstitial Pi and intracellular GSH. Magnetic resonance techniques, such as MRI and low-field EPR, are the methods of choice for noninvasive in vivo profiling of these TME parameters. MRI visualizes anatomical structures but lacks functional sensitivity. In contrast to MRI, EPR techniques provide functional sensitivity to...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was partially supported by NIH grants CA194013, CA192064 and U54GM104942. The WVCTSI is acknowledged for start-up to VVK, AB, and TDE. The authors thank Dr. M. Gencheva and K. Steinberger for the assistance with the illustrative experiments. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Materiały
| Name | Company | Catalog Number | Comments |
| L-band EPR spectrometer | Magnettech, Germany | L-band (1.2 GHz) electron paramagnetic resonance (EPR) spectrometer for collection in vitro and in vivo spectra of paramagnetic molecules | |
| Temperature & Gas Controller | Noxygen, Germany | Temperature & Gas Controller designed to control and adjust the temperature and gas composition | |
| Sonicator | Fisher Scientific | ||
| GSH (L-Glutathione reduced) | Sigma-Aldrich | G4251 | |
| MMTV-PyMT mice | In house | ||
| DMEM | Thermo Fisher Scientific | 11995065 | |
| Met-1 murine breast cancer cells | In house | ||
| C57Bl/6 wild type mice | Jackson Laboratory | ||
| Trypsin | Thermo Fisher Scientific | 25200056 | |
| Trypan Blue Exclusion Dye | Thermo Fisher Scientific | T10282 | |
| Ohmeda Fluotec 3 | |||
| Isoflurane (IsoFlo) | Abbott Laboratories | ||
| Sodium phosphate dibasic | Sigma-Aldrich | S9763 | |
| Sodium phosphate monobasic | sigma-Aldrich | S07051 | |
| Sodium Chloride | sigma-Aldrich | S7653 | |
| Hydrochloric acid | sigma-Aldrich | 320331 | |
| Sodium Hydroxide | sigma-Aldrich | S8045 | |
| Glucose | sigma-Aldrich | ||
| Glucose oxydase | sigma-Aldrich | ||
| Lauda Circulator E100 | Lauda-Brikmann | ||
| pH meter Orion | Thermo Scientific | ||
| LiNc-BuO probe | In house | The Octa-n-Butoxy-Naphthalocyanine probe was synthesizided according to ref 13 | |
| NR probe | In house | The Nitroxide probe was synthesizided according to ref 11 | |
| RSSR probe | In house | The di-Nitroxide probe was synthesizided according to ref 15 | |
| HOPE probe | In house | The monophoshonated Triarylmethyl probe was synthesizided according to ref 12 |
Odniesienia
- Siemann, D. W. . Tumor Microenvironment. , (2011).
- Tatum, J. L., et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 82 (10), 699-757 (2006).
- Brahimi-Horn, M. C., Chiche, J., Pouyssegur, J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 19 (2), 223-229 (2007).
- Haulica, A., Ababei, L. Comparative study of glycolytic activity in the erythrocytes of animals with chronic experimental hypoxia and with tumours. Neoplasma. 21 (1), 29-35 (1974).
- Matsumoto, K., et al. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res. 12 (8), 2455-2462 (2006).
- Estrela, J. M., Ortega, A., Obrador, E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 43 (2), 143-181 (2006).
- Voegtlin, C., Thompson, J. W. Glutathione content of tumor animals. J. Biol. Chem. 70, 801-806 (1926).
- Bobko, A. A., et al. Interstitial Inorganic Phosphate as a Tumor Microenvironment Marker for Tumor Progression. Sci Rep. 7, 41233 (2017).
- Gillies, R. J., Raghunand, N., Garcia-Martin, M. L., Gatenby, R. A. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag. 23 (5), 57-64 (2004).
- Gade, T. P., et al. Imaging intratumoral convection: pressure-dependent enhancement in chemotherapeutic delivery to solid tumors. Clin Cancer Res. 15 (1), 247-255 (2009).
- Bobko, A. A., et al. In vivo monitoring of pH, redox status, and glutathione using L-band EPR for assessment of therapeutic effectiveness in solid tumors. Magn Reson Med. 67, 1827-1836 (2012).
- Dhimitruka, I., Bobko, A. A., Eubank, T. D., Komarov, D. A., Khramtsov, V. V. Phosphonated Trityl Probe for Concurrent In Vivo Tissue Oxygen and pH Monitoring Using EPR-based Techniques. JACS. 135, 5904-5910 (2013).
- Pandian, R. P., Parinandi, N. L., Ilangovan, G., Zweier, J. L., Kuppusamy, P. Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radic Biol Med. 35 (9), 1138-1148 (2003).
- Bobko, A. A., Evans, J., Denko, N. C., Khramtsov, V. V. Concurrent Longitudinal EPR Monitoring of Tissue Oxygenation, Acidosis, and Reducing Capacity in Mouse Xenograft Tumor Models. Cell Biochem Biophys. 75, 247-253 (2017).
- Khramtsov, V. V., Yelinova, V. I., Glazachev Yu, I., Reznikov, V. A., Zimmer, G. Quantitative determination and reversible modification of thiols using imidazolidine biradical disulfide label. J Biochem Biophys Methods. 35 (2), 115-128 (1997).
- Roshchupkina, G. I., et al. In vivo EPR measurement of glutathione in tumor-bearing mice using improved disulfide biradical probe. Free Rad. Biol. Med. 45, 312-320 (2008).
- Khramtsov, V. V., Zweier, J. L., Hicks, R. . Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds. , 537-566 (2010).
- Bobko, A. A., Dhimitruka, I., Zweier, J. L., Khramtsov, V. V. Fourier Transform EPR of Trityl Radicals for Multifunctional Assessment of Chemical Microenvironment). Angew. Chem. Int. Edit. 53, 2735-2738 (2014).
- Martin, M. L., Martin, G. J., Delpuech, J. J. . Practical NMR spectroscopy. , (1980).
- Lin, E. Y., et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 163 (5), 2113-2126 (2003).
- Eubank, T. D., et al. Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res. 69 (5), 2133-2140 (2009).
- Khramtsov, V. V., et al. Quantitative determination of SH groups in low- and high-molecular-weight compounds by an electron spin resonance method. Anal Biochem. 182 (1), 58-63 (1989).
- Komarov, D. A., et al. Electron paramagnetic resonance monitoring of ischemia-induced myocardial oxygen depletion and acidosis in isolated rat hearts using soluble paramagnetic probes. Magnetic Resonance in Medicine. 68 (2), 649-655 (2012).
- Song, Y. G., Liu, Y. P., Liu, W. B., Villamena, F. A., Zweier, J. L. Characterization of the binding of the Finland trityl radical with bovine serum albumin. Rsc Advances. 4 (88), 47649-47656 (2014).
- Khramtsov, V. V., Bobko, A. A., Tseytlin, M., Driesschaert, B. Exchange Phenomena in the Electron Paramagnetic Resonance Spectra of the Nitroxyl and Trityl Radicals: Multifunctional Spectroscopy and Imaging of Local Chemical Microenvironment. Analyt. Chem. 89 (9), 4758-4771 (2017).
- Samouilov, A., et al. In Vivo Proton-Electron Double-Resonance Imaging of Extracellular Tumor pH Using an Advanced Nitroxide Probe. Analyt. Chem. 86 (2), 1045-1052 (2014).
- Goodwin, J., et al. In vivo tumour extracellular pH monitoring using electron paramagnetic resonance: the effect of X-ray irradiation. NMR Biomed. 27 (4), 453-458 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone