Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Observation and Analysis of Blinking Surface-enhanced Raman Scattering
W tym Artykule
Podsumowanie
This protocol describes the analysis of blinking surface-enhanced Raman scattering due to the random walk of a single molecule on a silver surface using power laws.
Streszczenie
From a single molecule at a silver nanoaggregate junction, blinking surface-enhanced Raman scattering (SERS) is observed. Here, a protocol is presented on how to prepare the SERS-active silver nanoaggregate, record a video of certain blinking spots in the microscopic image, and analyze the blinking statistics. In this analysis, a power law reproduces the probability distributions for bright events relative to their duration. The probability distributions for dark events are fitted by a power law with an exponential function. The parameters of the power law represent molecular behavior in both bright and dark states. The random walk model and the speed of the molecule across the entire silver surface can be estimated. It is difficult to estimate even when using averages, autocorrelation functions, and super-resolution SERS imaging. In the future, power law analyses should be combined with spectral imaging, because the origins of blinking cannot be confirmed by this analysis method alone.
Wprowadzenie
Surface-enhanced Raman scattering (SERS) is highly sensitive Raman spectroscopy from a noble metal surface. Since the Raman spectrum provides detailed information about molecular structure based on the sharp peak positions, through the vibrational modes of functional groups in the molecules, the information of a single molecule on a metal surface can be investigated using SERS1,2,3. From a silver nanoaggregate with an adsorbate at the single-molecule level, a blinking signal is observed1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16, and the spectrum fluctuates1,2,3,4,5,6,7,8,9,10,11,12,13,14. Blinking can be induced by a single molecule that randomly moves in and out of an enhanced electromagnetic (EM) field at a nanometer-sized silver nanoaggregate junction. Therefore, blinking is considered simple evidence for single-molecule detection, compared with a technique using a Poisson distribution of SERS intensities and a bi-analyte2,3,17. However, the detailed mechanisms of the blinking and fluctuating spectrum, which may strongly depend on molecular behavior on the Ag surface, are still controversial.
In previous studies, blinking SERS has been analyzed using the autocorrelation function, which can calculate the diffusion coefficient and concentration of molecules moving in and out of an enhanced EM field12,13,14. Moreover, a normalized standard deviation score, which represents instability in the total intensity, has been derived from the time profile of the signal15. However, these analytic approaches may be based on the behavior of a few molecules. In contrast, in a super-resolution imaging of blinking SERS, single-molecule behavior in an enhanced EM field can be identified16. However, these techniques can obtain such parameters only in an enhanced EM field. The random behavior of a single molecule within a wide range (for instance, in blinking SERS) can be represented as a power law rather than an average4,5,6,7,8,9,10,11, similar to blinking fluorescence from a single semiconductor quantum dot (QD)18,19. By using a power law analysis4,5,6,7,8,9,10,11, molecular behavior can be estimated in both the bright state (in the enhanced EM field) and dark state10; that is, the behavior of the molecule over the entire silver surface can be estimated.
For this technique, silver colloidal nanoaggregates are used4,5,6,7,8,9,10,11. These nanoaggregates show various localized surface plasmon resonance (LSPR) bands that strongly affect enhanced electromagnetic fields when they are excited at certain wavelengths. Thus, SERS-active silver nanoparticles exist in colloidal suspension, and some data can immediately be obtained. In the case of simple nanostructures, which have specific sizes, shapes, and arrangements, the LSPR dependence of SERS blinking can conceal other dependences7; namely, if the good or bad nanostructure to LSPR is used, the parameters will be constant, and the other dependences will therefore be hidden. Power law analysis has been used to discover various dependences of the blinking SERS from silver colloidal nanoaggregates4,5,6,7,8,9,10,11.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Sample Preparation
- Preparation of silver colloidal nanoparticles20
- To fabricate silver colloidal nanoparticles, dissolve 0.030 g of silver nitrate and 0.030 g of trisodium citrate dihydrate in 150 mL of water in a 200-mL round bottom flask.
- Combine the flask with a reflux (Dimroth) condenser.
- Stir the solution in the flask with a magnetic stirrer and stir bar. Then, heat the stirring solution in the flask in an oil bath at 150 °C for 60 min.
NOTE: The solution will turn yellow, then milky grey. - Cool the suspension at room temperature, and keep the suspension in the flask covered with aluminum foil in a refrigerator.
NOTE: The protocol can be paused at this point. Use the colloidal nanoparticles, after storing in a refrigerator, within one month.
- Preparation of sample for multi-colored blinking emission11
- To ready a microscope slide, wash a glass plate with soap by hand and rinse it with water.
- Add 0.1% poly-L-lysine aqueous solution to the glass plate, and remove the solution with a blower.
- Add the silver colloidal suspension to the glass plate, and remove the suspension with a blower.
- Enclose a drop area on the glass plate with a liquid blocker pen.
- Drop distilled water on the glass plate, and cover it with another glass plate to create a microscope slide and prevent water from evaporating.
- Preparation of sample for monotonous colored blinking SERS7,8,9,10
- To ready a microscope slide, wash a glass plate with soap by hand and rinse it with water.
- Mix the silver colloidal suspension with thiacyanine or thiacarbocyanine dye (25 or 4 µM, respectively) and a NaCl (10 mM) aqueous solution at a volume ratio of 2:1:1.
- Drop the sample suspension onto the glass plate, and remove the suspension with a blower.
- Enclose a drop area on the glass plate with a liquid blocker pen.
- Drop an aqueous solution of NaCl (1 M) on the glass plate to immobilize the silver nanoparticles, and cover it with another glass plate to create a microscope slide plate and prevent the solution from evaporating.
2. Observation of Blinking Silver Nanoparticles
- Illumination of sample
- Place the sample glass plate prepared using protocol 1.2 or 1.3 on the stage of an inverted microscope.
- Illuminate the sample glass plate using white light through a dark field condenser, and focus on various colored spots (blue, green, yellow, and red) on the glass plate using an objective lens (60X).
- Illuminate the sample glass plate using an attenuated beam, delivered at an angle of 30° relative to the sample surface, from a diode pumped solid state (DPSS) continuous-wave (cw) laser through an interference filter.
- To use laser illumination to observe the silver nanoaggregates as monotonous colored spots in a same-colored surrounding, move the laser illumination area to the center of the view, and focus on the spots on the glass plate by adjusting the stage in the z-direction.
- Observation of blinking
- Insert a long-pass filter after the objective lens, and illuminate the sample glass plate using a DPSS cw-laser beam delivered at an angle of 30° relative to the sample surface through an interference filter.
- Find the blinking spots as shown in Figure 1 (see also Figure S1 in the supplementary material) by moving the stage in the x- and y-directions.
- Record video of the blinking spots with the inverted microscope, coupled to a cooled digital charge-coupled device (CCD) camera, which has a time resolution of 61 - 120 ms, for 20 min.
3. Analysis of Blinking SERS
- Derivation of time profile from video
- In the software that controls the CCD camera, open the video file.
- To select the blinking spots and dark area, drag areas that separately cover regions with and without spots in the video image.
- To derive signal intensity time profiles from the blinking spots and dark areas in the video, select Temporal Analysis in Analysis, and click the Calculate in the Temporal Analysis window.
- Save the data as a text file.
- Analysis of the time profile
- Flatten a baseline of the time profile by subtracting the time profile from the dark area and/or fitting with a polynomial function, as shown in Figures 2A and 2B.
- Evaluate an averaged baseline intensity that consists of approximately 2000 points, Ibase, and a standard deviation of baseline intensities, σ, as shown in Figures 2C and 2D.
- Distinguish bright events from dark events using larger intensities than a threshold of Ibase + 3σ, and record the duration of each event. In Figure 3, for example, record the event from 0 to 3.5476 s as the dark event (with a duration of t = 3.5476 s), and record the event from 3.5476 to 4.0981 s as the bright event (with a duration of t = 0.5505 s). Repeat procedure as shown in Table 1.
- Count the number of bright and dark events for each duration, as expressed in the first and second lines of Table 2.
- Sum the number of events for each duration, except for events shorter than duration t. As expressed in the second and third lines of Table 2, for example, sum the number of events for each duration (except for the events for t = 0.0612 s) as 41 + 18 + 9 + …; the result equals the summation for t = 0.1223 s, i.e. 103.
- Divide the summations by each duration, and normalize them. As expressed in Table 2, for example, divide the summation for duration t = 0.0612 s by the duration 0.0612 s. The result is 3,351.5791. Then, divide the result by the total of the results in the fourth line in Table 2. The probability distribution is derived to be 0.64494.
- Plot the probability distributions for the bright events Pon(t) against their duration t in a logarithm-logarithm graph, and fit Log10Pon(t) by Log10(
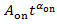 ) to deduce the power law exponent αon for a specific blinking spot. If Pon(t) is fitted by
) to deduce the power law exponent αon for a specific blinking spot. If Pon(t) is fitted by 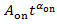 , the fitted line deviates from the plots at small values of Pon(t), as shown by the dotted line in Figure 4A.
, the fitted line deviates from the plots at small values of Pon(t), as shown by the dotted line in Figure 4A. - Plot the probability distributions for dark events Poff(t) against their duration t in a logarithm-logarithm graph, and fit Log10Poff(t) by Log10(
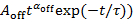 to deduce the power law exponent αoff and the truncation time τ from the same blinking spot. If Poff(t) is fitted by
to deduce the power law exponent αoff and the truncation time τ from the same blinking spot. If Poff(t) is fitted by 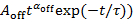 , the fitted curve deviates from the plots at small values of Poff(t).
, the fitted curve deviates from the plots at small values of Poff(t). - Repeat 3.2.1 to 3.2.8 for the other blinking spots in the video.
Access restricted. Please log in or start a trial to view this content.
Wyniki
From the silver nanoaggregates with poly-L-lysine prepared by protocol 1.2, multicolored blinking spots from SERS and surface-enhanced fluorescence are observed, as shown in Figure 111. In contrast, monotonous colored blinking spots from SERS were observed for the silver nanoaggregates with the dye molecules prepared by protocol 1.37,8,9,...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
From the silver nanoaggregate junction, SERS is emitted. Thus, we need to prepare nanoaggregates rather than colloidal nanoparticles, which are covered with citrate anions. Silver aggregates are formed from the salting out effect created by the addition of poly-L-lysine, which has -NH3+ and is the origin of the SERS, or Na+ cations from NaCl, as shown in Figure S2 of the supplementary material. Moreover, to illuminate the many spots in the wide area, the unfocused laser b...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The author has nothing to disclose.
Podziękowania
The author thanks Prof. Y. Ozaki (Kwansei Gakuin University) and Dr. T. Itoh (National Institute of Advanced Industrial Science and Technology) for their fruitful discussion of this work. This work was supported by KAKENHI (Grant-in-Aid for Scientific Research C) from the Ministry of Education, Culture, Sports, Science, and Technology (No. 16K05671).
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Silver nitrate, 99.8% | Wako | 194-00832 | |
| Trisodium citrate dihydrate, 99. % | Wako | 191-01785 | |
| Poly-L-lysine aqueous solution, 0.1% | Sigma-Aldrich | P8920 | |
| 3,3'-disulfopropylthiacyanine triethylamine | Hayashibara Biochemical Laboratories | NK-2703 | a kind of thiacyanine dyes |
| 3,3'-diethyl-5,5'-dichloro-9-methylthiacarbocyanine iodine salt | Hayashibara Biochemical Laboratories | SMP-9 | a kind of thiacarobocyanine dyes |
| Sodium chloride, 99.5% | Wako | 191-01665 | |
| Dimroth condenser | Iwaki | 61-9722-22 | perchased from AS ONE |
| Magnetic stirrer | Corning | DC-420D | |
| Oil bath | Advantech | OS-220 | |
| Glass plate | Matsunami | S-1112 | Microscope slide |
| Blower | Hozan | Z-288 | Air duster |
| Liquid blocker pen | Daido Sangyo | LIQUID BLOCKER (Super Pap Pen). Ready-to-use hydrophobic barrier pen designed for immunohistochemistry applications | |
| Inverted microscope | Olympus | IX-70 | |
| Objective lens | Olympus | LCPlanFl 60× | NA 0.7 |
| Dark field condenser | Olympus | U-DCD | NA 0.8–0.92 |
| Cooled digital CCD camera | Hamamatsu | ORCA-AG | controlled by software Aqua Cosmos |
| Software for the cooled digital CCD camera | Hamamatsu | AquaCosmos | used for also derivation of the time-profiles from the blinking spots in the video |
| Color CCD camera | ELMO | TNC-C920 | not used for analysis |
| DPSS laser | RGB laser system | NovaPro532-75 | λ = 532 nm; 60 mW (corresponds to a power density of 600 W/cm2) |
| Interference filter | Semrock | LL01-532-12.5 | |
| Long pass filter | Semrock | BLP01-532R-25 | |
| Software for the distinguishment and counting of the bright/dark events | home-maid | programmed by C++ | |
| Software for the fitting by a power law | LightStone | Origin6.1 |
Odniesienia
- Qian, X. M., Nie, S. M. Single-molecule and single-nanoparticle SERS: from fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 37, 912-920 (2008).
- Pieczonka, N. P. W., Aroca, R. F. Single molecule analysis by surfaced-enhanced Raman scattering. Chem. Soc. Rev. 37, 946-954 (2008).
- Kneipp, J., Kneipp, H., Kneipp, K. SERS -a single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 37, 1052-1060 (2008).
- Kitahama, Y., Ozaki, Y. Analysis of blinking SERS by a power law with an exponential function. Frontiers of Surface-Enhanced Raman Scattering: Single-Nanoparticles and Single Cells. , Wiley. Chichester. Chapter 6 (2014).
- Kitahama, Y. Truncated Power Law Analysis of Blinking SERS. Frontiers of Plasmon Enhanced Spectroscopy Volume 1 (ACS Symposium series Vol. 1245). , American Chemical Society. Washington DC. Chapter 4 (2016).
- Bizzarri, A. R., Cannistraro, S. Lévy Statistics of Vibrational Mode Fluctuations of Single Molecules from Surface-Enhanced Raman Scattering. Phys. Rev. Lett. 94, 068303(2005).
- Kitahama, Y., Tanaka, Y., Itoh, T., Ozaki, Y. Power-law analysis of surface-plasmon-enhanced electromagnetic field dependence of blinking SERS of thiacyanine or thiacarbocyanine adsorbed on single silver nanoaggregates. Phys. Chem. Chem. Phys. 13, 7439-7448 (2011).
- Kitahama, Y., Tanaka, Y., Itoh, T., Ozaki, Y. Analysis of excitation laser intensity dependence of blinking SERRS of thiacarbocyanine adsorbed on single silver nanoaggregates by using a power law with an exponential function. Chem. Commun. 47, 3888-3890 (2011).
- Kitahama, Y., Enogaki, A., Tanaka, Y., Itoh, T., Ozaki, Y. Truncated power law analysis of blinking SERS of thiacyanine molecules adsorbed on single silver nanoaggregates by excitation at various wavelengths. J. Phys. Chem. C. 117, 9397-9403 (2013).
- Kitahama, Y., Araki, D., Yamamoto, Y. S., Itoh, T., Ozaki, Y. Different behaviour of molecules in dark SERS state on colloidal Ag nanoparticles estimated by truncated power law analysis of blinking SERS. Phys. Chem. Chem. Phys. 17, 21204-21210 (2015).
- Kitahama, Y., Nagahiro, T., Tanaka, Y., Itoh, T., Ozaki, Y. Analysis of blinking from multicoloured SERS-active Ag colloidal nanoaggregates with poly-L-lysine via truncated power law. J. Raman. Spectrosc. 48, 570-577 (2017).
- Habuchi, S., et al. Single-Molecule Surface Enhanced Resonance Raman Spectroscopy of the Enhanced Green Fluorescent Protein. J. Am. Chem. Soc. 125, 8446-8447 (2003).
- Weiss, A., Haran, G. Time-Dependent Single-Molecule Raman Scattering as a Probe of Surface Dynamics. J. Phys. Chem. B. 105, 12348-12354 (2001).
- Emory, S. R., Jensen, R. A., Wenda, T., Han, M., Nie, S. Re-examining the origins of spectral blinking in single-molecule and single-nanoparticle SERS. Faraday Discuss. 132, 249-259 (2006).
- Itoh, T., Iga, M., Tamaru, H., Yoshida, K., Biju, V., Ishikawa, M. Quantitative evaluation of blinking in surface enhanced resonance Raman scattering and fluorescence by electromagnetic mechanism. J. Chem. Phys. 136, 024703(2012).
- Willets, K. A. Super-resolution imaging of SERS hot spots. Chem. Soc. Rev. 43, 3854-3864 (2014).
- Dieringer, J. A., Lettan, R. B., Scheidt, K. A., Van Duyne, R. P. A Frequency Domain Existence Proof of Single-Molecule Surface-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 129, 16249-16256 (2007).
- Cichos, F., von Borczyskowski, C., Orrit, M. Power-law intermittency of single emitters. Curr. Opin. Colloid Interface Sci. 12, 272-284 (2007).
- Tang, J., Marcus, R. A. Mechanisms of fluorescence blinking in semiconductor nanocrystal quantum dots. J. Chem. Phys. 123, 054704(2005).
- Lee, P. C., Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 86, 3391-3395 (1982).
- Krichevsky, O., Bonnet, G. Fluorescence correlation spectroscopy: the technique and its applications. Rep. Prog. Phys. 65, 251-297 (2002).
- Hess, S. T., Huang, S., Heikal, A. A., Webb, W. W. Biological and Chemical Applications of Fluorescence Correlation Spectroscopy: A Review. Biochemistry. 41, 697-705 (2002).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone