Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Total Internal Reflection Absorption Spectroscopy (TIRAS) for the Detection of Solvated Electrons at a Plasma-liquid Interface
W tym Artykule
Podsumowanie
This article presents a total internal reflection absorption spectroscopy (TIRAS) method for measuring short-lived free radicals at a plasma-liquid interface. In particular, TIRAS is used to identify solvated electrons based on their optical absorbance of red light near 700 nm.
Streszczenie
The total internal reflection absorption spectroscopy (TIRAS) method presented in this article uses an inexpensive diode laser to detect solvated electrons produced by a low-temperature plasma in contact with an aqueous solution. Solvated electrons are powerful reducing agents, and it has been postulated that they play an important role in the interfacial chemistry between a gaseous plasma or discharge and a conductive liquid. However, due to the high local concentrations of reactive species at the interface, they have a short average lifetime (~1 µs), which makes them extremely difficult to detect. The TIRAS technique uses a unique total internal reflection geometry combined with amplitude-modulated lock-in amplification to distinguish solvated electrons' absorbance signal from other spurious noise sources. This enables the in situ detection of short-lived intermediates in the interfacial region, as opposed to the bulk measurement of stable products in the solution. This approach is especially attractive for the field of plasma electrochemistry, where much of the important chemistry is driven by short-lived free radicals. This experimental method has been used to analyze the reduction of nitrite (NO2-(aq)), nitrate (NO3-(aq)), hydrogen peroxide (H2O2(aq)), and dissolved carbon dioxide (CO2(aq)) by plasma-solvated electrons and deduce effective rate constants. Limitations of the method may arise in the presence of unintended parallel reactions, such as air contamination in the plasma, and absorbance measurements may also be hindered by the precipitation of reduced electrochemical products. Overall, the TIRAS method can be a powerful tool for studying the plasma-liquid interface, but its effectiveness depends on the particular system and reaction chemistry under study.
Wprowadzenie
Plasma-liquid interactions represent an area of growing interest in the plasma science and engineering community. The complex interface between plasmas and liquids, which contains a variety of highly reactive free radicals, has found applications in many areas including analytical chemistry, plasma medicine, water and wastewater treatment, and nanomaterial synthesis1,2,3,4,5,6. While there are various configurations that can be used to bring a plasma in contact with a liquid7, perhaps the simplest is the plasma analog of an electrolytic cell, where one of the standard metal electrodes is replaced with a plasma or gas discharge8. The plasma electrochemical cell consists of a reactor vessel, a submerged metal electrode, and a plasma discharge, which can function as either the cathode or anode (or both). When the plasma discharge is used as a cathode, gas-phase electrons generated in the plasma are injected into the solution. After the electrons enter the solution, their kinetic energy dissipates on the timescale of femtoseconds9,10,11 primarily through inelastic scattering off the solvent molecules. Once the electrons have reached a near-thermal kinetic energy, they trap and solvate in a cavity formed by surrounding solvent molecules. Depending on the solvent and temperature, these "solvated" electrons may be stable until they react with some reducible species in the solution or with another solvated electron. In aqueous solution, solvated electrons are also referred to as hydrated electrons12.
This process of solvation has long been known, and the detection of hydrated electrons generated by procedures such as pulse radiolysis or flash photolysis has been studied since the 1960s13,14,15. In traditional radiolysis and photolysis, the solvated electrons are produced via ionization of the solvent molecules; however, electrons solvated at the plasma-liquid interface are injected from the gaseous plasma16. Previous experiments have determined that hydrated electrons absorb red light near 700 nm13,14,17, which allows them to be experimentally studied via optical absorption spectroscopy. Other experiments have measured their diffusion constants, their reaction rates with hundreds of chemical species, their radius of gyration, and their charge mobility, among other properties of interest12,18.
Within the literature, several methods to detect solvated electrons have been reported, which can be separated mainly into two types: bulk dosimetry, where solvated electron presence is inferred from the bulk chemical analysis of their reaction products, and direct transient absorption spectroscopy, where the electrons' absorbance is measured as the reaction takes place. The latter category, upon which the methodology presented here is based, has the advantage of direct and instant evidence, as well as the ability to monitor intermediate reactions.
The rationale behind the development of the total internal reflection absorption spectroscopy (TIRAS) methodology was to directly study the role of solvated electrons at the plasma-liquid interface. The reflection geometry was chosen, because the production of solvated electrons using a plasma discharge, as opposed to methods like radiolysis or photolysis, occurs at the interface between the plasma and the liquid. When a probe laser grazes the surface at a shallow angle of incidence, it is totally reflected back into the solution and out into a detector, less the small amount of light absorbed by the electrons. With no light escaping into the plasma, the experimental technique only measures free radicals in the liquid phase, just beneath the interface, and is thus a highly sensitive interfacial measurement technique. Additionally, the total internal reflection phenomenon has the advantage of eliminating noise from the changing of partial reflections due to surface fluctuations, which could otherwise dominate the signal.
The TIRAS protocol outlined in this article has three essential features. The first is a plasma electrochemical cell, which consists of a transparent glass beaker with two optical windows at angles of approximately 20° facing downward and a controlled headspace of argon gas. The second feature is the optical measurement system, which includes a diode laser, an optical cage, and a photodiode detector. The laser provides the light that is absorbed by the solvated electrons, and is mounted in line with an adjustable iris and a 50 mm lens in an optical cage. This arrangement is mounted on a goniometer, which allows it to be rotated to a desired angle of incidence. The intensity of the transmitted light is then measured by the photodetector, which consists of a large area photodiode wired in a reverse-bias leakage circuit. Finally, because of their high reactivity, solvated electrons only penetrate ~10 nm into the solution, which yields an extremely small optical absorption signal of ~10-5 optical density. To ensure a sufficiently high signal-to-noise ratio, the third essential component is a lock-in amplification system, which consists of a plasma switching circuit and a lock-in amplifier. In the switching circuit, a solid-state relay circuit modulates the plasma current between a high and a low value at a carrier frequency of 20 kHz set by a function generator. This, in turn, also modulates the solvated electron concentration at the interface and their optical absorbance. The lock-in amplifier then takes the signal from the photodetector and filters all noise outside the carrier frequency.
The TIRAS method has great potential to reveal important chemical processes in plasma-liquid experiments, particularly in plasma electrochemistry. The reduction and oxidation pathways are primarily driven by a variety of short-lived radicals at the plasma-liquid interface, and the detection of the species is extremely important for understanding the interfacial chemistry. The in situ monitoring capabilities of TIRAS will help establish a greater understanding of the important electron-driven reactions involved at the plasma-liquid interface. TIRAS, for example, makes the measurement of reaction rates possible in the presence of electron scavengers. Previous studies have focused on the reduction of NO2-(aq), NO3-(aq), and H2O2(aq) scavengers dissolved in the aqueous solution16, as well as the reduction of dissolved CO2(aq)19. Other studies have focused on the effect of the plasma carrier gas on plasma-solvated electron chemistry20.
Protokół
1. Constructing the Experimental Setup
Note: To run this experiment, assemble a system consisting of a plasma reactor where the reaction will take place, optical components for absorbance measurements, and the electronic lock-in amplification system to process the signal.
- Construct the plasma electrochemical cell.
- Manufacture a reactor cell consisting of a transparent glass vessel, 50.8 mm (2 in) in diameter, with two optical windows at angles of approximately 20° down from the normal plane.
- Construct a non-permeable lid containing four orifices, which will be used to introduce the plasma electrode, a platinum anode, and a small hose for flushing the head space with argon (Ar).
Note: The fourth orifice will allow Ar to vent from the head space. - Form the anode by attaching a piece of platinum foil to a stainless-steel rod.
- Form the cathode by sharpening the end of a 1.58 mm (1/16 in) outer diameter, 0.178 mm (0.007 in) inner diameter stainless steel capillary. Connect an Ar hose to the blunt end of the capillary using appropriate fittings.
- Construct the optical measurement apparatus.
- Construct a laser source consisting of a 670 nm diode laser, an adjustable iris, and a 50 mm lens all mounted in a 30 mm optical cage system. Mount the cage to a goniometer, so that the entire system may be rotated at an angle of 19° with the laser directed into one of the optical windows of the electrochemical cell.
Note: Other laser wavelengths may also be used, see Ref 16. - Construct a photodetector consisting of a large area photodiode wired into a reverse-bias leakage circuit. Mount the photodetector to a goniometer so that it may receive reflected light through the optical window of the electrochemical cell opposite to the laser source. Additionally, mount to the detector a bandpass filter corresponding to the laser wavelength.
Note: A diagram of the photodetector circuit can be seen in Figure 1. For such a circuit, the output voltage is directly proportional to the laser intensity.
- Construct a laser source consisting of a 670 nm diode laser, an adjustable iris, and a 50 mm lens all mounted in a 30 mm optical cage system. Mount the cage to a goniometer, so that the entire system may be rotated at an angle of 19° with the laser directed into one of the optical windows of the electrochemical cell.
- Construct the plasma switching circuit and lock-in amplifier circuit.
Note: The electronic components include a high voltage power supply, a high voltage switching circuit, a function generator, a voltmeter, and a lock-in amplifier.- Connect the high voltage power supply so that it may apply a direct current (DC) bias of approximately -2.5 kV between the plasma electrode and the anode to generate the plasma.
- Use a custom-built switching circuit to modulate the plasma current between high and low values at a carrier frequency of 20 kHz.
Note: A schematic of the circuit is shown in Figure 2. The high-voltage power supply drives the current through two ballast resistors connected in parallel. While a small current continuously flows through the 3 MΩ resistor, the current through the 220 kΩ is continuously switched by an insulated gate bipolar junction transistor (IGBT). The function generator produces the 20 kHz driving frequency, and is connected to the IGBT through an optical isolator, which isolates the high-voltage power supply from the function generator. - Integrate the lock-in amplifier to allow the output of the photodetector to be connected to it.
Note: The lock-in amplifier will filter noise outside of the 20 kHz band. Its outputs, namely the amplitude and phase of the absorbance signal, should be recorded by a computer. An in-house program was used to record the output.
2. Prepare NaClO4 Solution as a Conductive Background Electrolyte
- To prepare a 0.163 M NaClO4 solution, which is the concentration used in this experiment, dissolve 10 g of NaClO4 in 500 mL of deionized water. Note: NaClO4 was chosen as an electrolyte because it does not react with solvated electrons. The concentration of 0.163 M is only representative, and different concentrations can be used, typically on the order of 0.001-0.1 M.
- Pour 60 mL of NaClO4 into the plasma reactor.
3. Prepare Setup for Measurements
- Prepare the electrochemical cell set-up and purge the reactor.
- Insert the anode through the appropriate orifice into the non-permeable lid. Partially submerge the anode beneath the surface of the solution.
- Connect the plasma electrode (cathode capillary) to a mass flow meter connected to an Ar tank and insert the capillary through the lid. Suspend the tip of the capillary approximately 1-2 mm above the surface of a solution. Use a camera to measure the distance between the capillary and the liquid surface.
- Connect the small hose to a mass flow meter connected to an Ar tank, and insert the hose through one of the orifices in the lid; this forms the purge line.
- With the cathode, anode, and Ar purge line in place, secure the non-permeable lid to the top of the reactor cell.
- Turn on the Ar flow through the purge line to approximately 250 cm3/min for at least 5 minutes to flush out air from the headspace of the plasma reactor.
Note: The flow rate and the duration of applied flow depend on the volume of the reactor and the volume of solution in the reactor; the values used here are representative. Failure to flush out air will lead to side reactions driven by dissolved oxygen in the solution (see Ref. 21).
- Align the measurement.
- Set the flow of Ar through the plasma electrode to roughly 10 cm3/min. Align the reactor so that the laser hits the plasma-liquid interface. Do this by observing light being scattered off the dimple by the flow of Ar gas.
- Close the flow of Ar to the plasma electrode and wait for the laser spot to return to its normal size. Once this happens, align the photodetector so that the laser hits the center of the detector.
- Measure baseline signal intensity and prepare electrical system.
- Connect the photodetector output to a voltmeter and measure the voltage given by the laser. Record this value, as it will be used later to normalize the measured absorbance signal.
Note: This value is directly proportional to the incident intensity I0. - After measuring the incident intensity, disconnect the cable from the photodetector and connect it to the input of the lock-in amplifier to lock with the signal at the 20 kHz carrier frequency.
- Connect the transistor-transistor logic output of the function generator to the frequency input of the lock-in amplifier.
- Make sure the lock-in amplifier, function generator, and high-voltage power supply are turned on.
Note: The experiment is now ready to be initiated.
- Connect the photodetector output to a voltmeter and measure the voltage given by the laser. Record this value, as it will be used later to normalize the measured absorbance signal.
4. Start Experiment and Data Collection
Note: An in-house program is used for data collection. Additionally, this system is automated to ensure precision and reduce human error. The underlying process of this automation is described in the following steps.
- Turn on the laser and begin measuring absorbance. Allow time for the noise to be integrated down to a sufficiently low level.
- Set the high-voltage power supply to a voltage difference of approximately -2.5 kV to ignite the plasma.
Note: A photograph of the plasma discharge at a distance of 1 mm from the liquid surface is shown in Figure 3. - Wait approximately half a minute for the amplitude measured by the lock-in amplifier to reach a steady-state and, thereafter, record the signal for approximately 2 min.
- Turn off the laser and wait for the laser signal to reach a steady-state. Subsequently, measure the absorbance for half a minute.
Note: This measurement will be subtracted from the previous absorbance measurements to account for the noise from the plasma. - Turn off the plasma by switching off the high-voltage power supply.
- To repeat an experiment, make sure the plasma electrode is still aligned. To do this, retract the plasma electrode at least 1 cm and then open the flow of Ar through the capillary. Repeat steps 3.2 to 4.5.
- If no more experiments will be repeated, turn off all electronic instruments, take off the lid from the electrochemical cell, and dispose of the NaClO4 appropriately.
5. Data Analysis
Note: Output from the lock-in amplifier contains information about the amplitude R and phase ϕ of the 20 kHz absorbance signal. This can be represented by cosine and sine components, X and Y, respectively. Because the lock-in amplifier measures the modulated amplitude of the signal between the high and low currents set at step 1.3.2, the X and Y represent the differences between these two signals, and are used to measure the difference of absorbance between the low and high states, ΔI.
- To properly analyze the data, normalize the X and Y time vectors by dividing them by the incident intensity voltage I0.
Note: This voltage, which was measured in Step 3.3.1, is directly proportional to the optical intensity, as are the signal components X and Y. Therefore, dividing X and Y by this value should yield dimensionless vectors representing the in-phase and out-of-phase components of the 20 kHz absorbance signal. - Obtain the average of X/I0 and Y/I0 from the moment steady-state was reached after the plasma was turned on until the laser was turned off.
Note: Figure 4 shows the normalized absorbance RMS magnitude R measured throughout the experiment. The plasma was switched on after 30 s, the absorbance signal reached steady-state at a time of 50 s, and the plasma was switched off at a time of 150 s. - Likewise, obtain the average of X/I0 and Y/I0 from the moment the laser was turned off until the plasma was turned off to obtain the cosine and sine components of the plasma noise. Note: In Figure 4, the plasma was turned off at a time of 150 s, and the noise measurement was the average absorbance detected from a time of 170 s to 200 s.
- To obtain the cosine and sine components of the absorbance signal, subtract the averages obtained in step 5.3 from the averages obtained in 5.2.
- To calculate the true absorbance, calculate the square root of the sum of the squares of the X and Y components of the signal obtained in step 5.4 as it is shown in Equation 1.
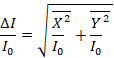 (1)
(1)
6. Extraction of Parameters
- Calculate the concentration of solvated electrons in the solution by assuming a steady state between the rate at which solvated electrons are introduced into the solution by the plasma, and the rate at which they are consumed.
Note: The consumption of solvated electrons, in the absence of other reactions, occurs through the second order recombination of electrons as shown in Equation 2.
 (2)
(2)
- Use Beer's Law, as shown in Equation 3, to find the concentration of the solvated electrons as a function of an unknown penetration depth l, where ε is the molar extinction coefficient, and θ is the angle of incidence (defined as 19° in step 1.2).
Note: The extinction coefficient of the solvated electron is ~ 19,000 L mol-1 cm-1 as described in Ref. 18.
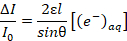 (3)
(3) - To extrapolate the unknown penetration depth, combine Equation 3 with Equation 4, which assumes electrons are introduced at a rate proportional to the current density of the plasma, where k is the reaction constant of the second order recombination, q is the elementary charge, j is the current density, and NA is Avogadro's number.
Note: A factor of 2 is included in Equation 4 to denote that two electrons are consumed by the reaction in Equation 2.
 (4)
(4)
The penetration length l and concentration [(e-)aq] can be determined from the measured signal using Equations 3 and 4.
- Use Beer's Law, as shown in Equation 3, to find the concentration of the solvated electrons as a function of an unknown penetration depth l, where ε is the molar extinction coefficient, and θ is the angle of incidence (defined as 19° in step 1.2).
7. Reaction Rate Estimation
Note: When electrons solvate in a solution with unreactive electrolytes such as NaClO4, solvated electrons are only consumed by the reaction in Equation 2. However, solvated electrons have the capacity to reduce a wide variety of cations, anions, and neutral species. When any of these electron scavengers are dissolved in the aqueous solution, they react with the solvated electrons. This lowers their equilibrium concentration and leads to a reduction of the absorbance detected, which allows the TIRAS methodology to estimate the reaction rate constants of these reactions. When a new reaction is introduced, the rate balance becomes:
 (5)
(5)
where [(S)aq] is the concentration of the electron scavenger in the solution, and k2 is the reaction rate constant associated with its reaction. However, if the scavenger concentration is sufficiently large, Equation 5 can be simplified to:
 (6)
(6)
Equation 3 can be then combined with Equation 6 to obtain a relationship between absorbance and the scavenger concentration.
 (7)
(7)
- To measure the reaction rate constant of solvated electrons with an electron scavenger, start by dissolving the scavenger in the NaClO4 solution, which was prepared in step 1.
Note: The non-reactive NaClO4 in the solution ensures a solution conductivity high enough for plasma stability. The concentration of the scavenger should be high enough to compete with the second order recombination, otherwise, the reaction will not take place. - Repeat steps 2.2 to 6.1.2 with different scavenger concentrations, and measure the difference in absorbance calculated in step 5.5, with respect to the pure NaClO4 solution.
- Make a plot of the absorbance as a function of [(S)aq]-1.
Note: If the scavenger concentration is sufficiently large for Equation 6 to be valid, plotting the absorbance as a function of [(S)aq]-1 will yield a straight line with a slope dependent on the rate constant k2. - Extrapolate the reaction rate constant k2 from the slope of the line.
Note: For additional details, as well as examples of how this was applied to the reactions of NO2-(aq), NO3-(aq), and H2O2(aq), CO2(aq) with solvated electrons, see Ref. 16,19.
Wyniki
As mentioned in step 5 of the procedure, this experiment measures the cosine and sine components of the absorbance signal, the phase angle between them, and the magnitude of the signal. A plot of the magnitude of the signal and its two components is shown in Figure 4.
Occasionally, there will be measurements which may be not optimal or even unusable. This may be due to a misalignment of the laser wi...
Dyskusje
The results show that the measurement of absorbance of light at the plasma-liquid interface is an effective method to detect and measure the concentration of plasma-solvated electrons in an aqueous solution. The subsequent measurement at different wavelengths results in the measurement of the absorption spectrum. Though this experiment was done in an aqueous NaClO4 solution, the methodology should be valid for a great variety of other liquids, provided that electrons can solvate in the liquid.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by the U.S. Army Research Office under Award Numbers W911NF-14-1-0241 and W911NF-17-1-0119. DMB is supported by the U.S. Department of Energy Office of Science, Office of Basic Energy Sciences under Award Number DE-FC02- 04ER1553.
Materiały
| Name | Company | Catalog Number | Comments |
| Function Generator | Protek | B8055 | |
| Lock-in Amplifier | Stanford Research Systems | SR830 | |
| High-Voltage Power Supply | Stanford Research Systems | PS325 | |
| Photodetector | Self-built | ||
| Flowmeter | Key Instruments | 60310 R5 | |
| Flow controller | Omega Engineering | FMA 5400A/5500A | |
| Camera | Dino-lite | Dinocapture 2.0 | |
| Voltmeter | Amprobe | AM-510 | |
| Optical Cage System | Thorlabs | 30 mm cage system | |
| Goniometers | Thorlabs | RP01 - Ø2 | Manual rotation stage |
| Diode lasers | Thorlabs | ||
| Electrochemical cell | Adams & Chittenden Scientific Glass | Custom-made product | |
| Stainless steel capillary | Restek | 0.007 in. ID | |
| SHV Coax Cable | SRS | Custom-made product | |
| Sodium Perchlorate | Sigma-Aldrich | ACS reagent, ≥98.0% | |
| Argon | Airgas | AR UHP300 | Ultra-high purity |
| LabVIEW | National Instruments | Software used to generate in-house program used to collect data |
Odniesienia
- Smoluch, M., Mielczarek, P., Silberring, J. Plasma-based ambient ionization mass spectrometry in bioanalytical sciences. Mass Spectrom. Rev. 35 (1), 22-34 (2015).
- Jamroz, P., Greda, K., Pohl, P. Development of direct-current, atmospheric-pressure, glow discharges generated in contact with flowing electrolyte solutions for elemental analysis by optical emission spectrometry. Trends Anal. Chem. 41, 105-121 (2012).
- Foster, J. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas. 24, (2017).
- Kong, M. G., et al. Plasma medicine: an introductory review. New J. Phys. 11, (2009).
- Chen, Q., Li, J., Li, Y. A review of plasma-liquid interactions for nanomaterial synthesis. J. Phys. D: Appl. Phys. 48, (2015).
- Mariotti, D., Patel, J., Svrcek, V., Maguire, P. Plasma-liquid interactions at atmospheric pressure for nanomaterials synthesis and surface engineering. Plasma Processes Polym. 9 (11-12), 1074-1085 (2012).
- Bruggeman, P. J., et al. Plasma-liquid interactions: a review and roadmap. Plasma Sources Sci. Technol. 25 (5), (2016).
- Rumbach, P., Go, D. B. Perspectives on plasmas in contact with liquids for chemical processing and materials synthesis. Top. Catal. , (2017).
- Mozumder, A. . Fundamentals of Radiation Chemistry. , (1999).
- Kai, T., Yokoya, A., Ukai, M., Fujii, K., Higuchi, M., Watanabe, R. Dynamics of low-energy electrons in liquid water with consideration of coulomb interaction with positively charged water molecules induced by electron collision. Radiat. Phys. Chem. 102, 16-22 (2014).
- Kai, T., Yokoya, A., Ukai, M., Fujii, K., Watanabe, R. Thermal equilibrium and prehydration processes of electrons injected into liquid water calculated by dynamic Monte Carlo method. Radiat. Phys. Chem. 115, 1-5 (2015).
- Hart, E. J., Anbar, M. . The hydrated electron. , (1970).
- Hart, E. J., Boag, J. W. Absorption spectrum of the hydrated electron in water and in aqueous solutions. J. Am. Chem. Soc. 84 (21), 4090-4095 (1962).
- Boag, J. W., Hart, E. J. Absorption spectra in irradiated water and some solutions. Nature. 197 (4862), 45-47 (1963).
- Matheson, M. S., Mulac, W. A., Rabani, J. Formation of the hydrated electron in the flash photolysis of aqueous solutions. J. Phys. Chem. 67 (12), 2613-2617 (1963).
- Rumbach, P., Bartels, D. M., Sankaran, R. M., Go, D. B. The solvation of electrons by an atmospheric-pressure plasma. Nat. Commun. 6 (7248), (2015).
- Anbar, M., Hart, E. J. The effect of solvent and of solutes on the absorption spectrum of solvated electrons. J. Phys. Chem. 69 (4), 1244-1247 (1965).
- Buxton, G. V., Greenstock, C. L., Helman, W. P., Ross, A. B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O-) in aqueous solution. J. Phys. Chem. Ref. Data. 17 (2), 513-886 (1988).
- Rumbach, P., Xu, R., Go, D. B. Electrochemical production of oxalate and formate from CO2 by solvated electrons produced using an atmospheric-pressure plasma. J. Electrochem. Soc. 163 (10), 1157-1161 (2016).
- Rumbach, P., Bartels, D. M., Sankaran, R. M., Go, D. B. The effect of air on solvated electron chemistry at a plasma/liquid interface. J. Phys. D: Appl. Phys. 48 (42), (2015).
- Rumbach, P., Witzke, M., Sankaran, R. M., Go, D. Decoupling interfacial reactions between plasmas and liquids: Charge transfer vs plasma neutral reactions. J. Am. Chem. Soc. 135, 16264-16267 (2013).
- Richmonds, C., Sankaran, R. M. Plasma-liquid electrochemistry: Rapid synthesis of colloidal metal nanoparticles by microplasma reduction of aqueous cations. Appl. Phys. Lett. 93, (2008).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone