Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
In Vitro Rearing of Solitary Bees: A Tool for Assessing Larval Risk Factors
W tym Artykule
Podsumowanie
Fungicide sprays on flowering plants may expose solitary bees to high concentrations of pollen-borne fungicide residues. Using laboratory-based experiments involving in vitro-reared bee larvae, this study investigates the interactive effects of consuming fungicide-treated pollen derived from host and non-host plants.
Streszczenie
Although solitary bees provide crucial pollination services for wild and managed crops, this species-rich group has been largely overlooked in pesticide regulation studies. The risk of exposure to fungicide residues is likely to be especially high if the spray occurs on, or near host plants while the bees are collecting pollen to provision their nests. For species of Osmia that consume pollen from a select group of plants (oligolecty), the inability to use pollen from non-host plants can increase their risk factor for fungicide-related toxicity. This manuscript describes protocols used to successfully rear oligolectic mason bees, Osmia ribifloris sensu lato, from egg to prepupal stage within cell culture plates under standardized laboratory conditions. The in vitro-reared bees are subsequently used to investigate the effects of fungicide exposure and pollen source on bee fitness. Based on a 2 × 2 fully crossed factorial design, the experiment examines the main and interactive effects of fungicide exposure and pollen source on larval fitness, quantified by prepupal biomass, larval developmental time, and survivorship. A major advantage of this technique is that using in vitro-reared bees reduces natural background variability and allows the simultaneous manipulation of multiple experimental parameters. The described protocol presents a versatile tool for hypotheses testing involving the suite of factors affecting bee health. For conservation efforts to be met with significant, lasting success, such insights into the complex interplay of physiological and environmental factors driving bee declines will prove to be critical.
Wprowadzenie
Given their role as the dominant group of insect pollinators1, the global loss in bee populations poses a threat to food security and ecosystem stability2,3,4,5,6,7. The declining trends in both managed and wild bee populations have been attributed to several shared risk factors including habitat fragmentation, emerging parasites and pathogens, loss of genetic diversity, and the introduction of invasive species3,4,7,8,9,10,11,12. In particular, the dramatic increase in the use of pesticides, (e.g., neonicotinoids) has been directly linked to detrimental effects among bees13,14,15. Several studies have shown that the synergism between neonicotinoids and ergosterol-biosynthesis-inhibiting (EBI) fungicides can lead to high mortality across multiple bee species16,17,18,19,20,21,22. Nevertheless, fungicides, long considered to be 'bee-safe', continue to be sprayed on in-bloom crops without much scrutiny23. Foraging bees have been documented to routinely bring back pollen loads contaminated with fungicide residues24,25,26. The consumption of such fungicide-ladenpollen can cause high mortality among larval bees27,28,29,30, and a suite of sub-lethal effects among adult bees16,31,32,33,34. A recent study suggests that fungicides may cause bee losses by altering the microbial community within hive-stored pollen, thereby disrupting the critical symbioses between bees and pollen-borne microbes35.
Although solitary bees are vital for the pollination of several wild and agricultural plants36,37,38, this diverse group of pollinators has received much less attention in pesticide monitoring studies. The nest of an adult solitary female contains 5-10 sealed brood chambers, each stocked with a finite mass of maternally-collected pollen and nectar, and a single egg39. After hatching, the larvae rely on the allocated pollen provision, and the associated pollen-borne microbiota to obtain adequate nutrition40,41. Because they lack the benefits of a social lifestyle, solitary bees may be more vulnerable to pesticide exposure42. For instance, while deficits in social bees following a spray may be compensated to some extend by workers and newly emerging brood, the death of a single adult solitary female ends all reproductive activity43. Such differences in susceptibility highlight the need to incorporate diverse bee taxa in ecotoxicological studies to ensure adequate protection for managed and wild bees alike. However, aside from a handful of studies, investigations into the effects of fungicide exposure has primarily focused on social bees18,23,32,44,45,46,47,48,49.
Solitary bees belonging to genus Osmia (Figure 1) have been used worldwide as efficient pollinators of several important fruit and nut crops39,50,51,53,53. As with other managed pollinator groups24,54,55,56,57,58, adult Osmia bees are routinely exposed to fungicides sprayed on in-bloom crops44. Adult females foraging on recently sprayed crops may collect and stock their brood chambers with fungicide-laden pollen, which later forms the sole diet for the developing larvae. Consuming the contaminated pollen provisions can subsequently expose the larvae to fungicide residues42. The risk of exposure may be higher among oligolectic species that forage only on a few closely related host plants59,60,61. Certain megachilid bees, for example, appear to preferentially forage for low-quality Asteraceae pollen, as a means of reducing parasitism62. However, the extent to which fungicides impact larval fitness among oligolectic solitary bees has not been empirically quantified. The goal of this study is to develop a protocol to test the main and interactive effects of fungicide exposure and pollen source on the fitness of in vitro reared solitary bees. To investigate, eggs of O. ribifloris sensu lato (s.l.) can be obtained commercially (Table of Materials). This population is ideal because of its importance as a native pollinator, and its strong predilection for the nectar-rich Mahonia aquifolium (Oregon grape) found within the region53,63,64 (Figure 2).

Figure 1. A high-resolution photo of an adult Osmia ribifloris. Photo credit Dr. Jim Cane, Research Entomologist, USDA-ARS Please click here to view a larger version of this figure.

Figure 2. Phragmite nesting reeds of Osmia ribifloris (s.l.) with a nesting female in the foreground. Chamber partitions and terminal plugs for the reeds are constructed from masticated leaves. Photo credit Mr. Kimball Clark, NativeBees.com Please click here to view a larger version of this figure.
The first objective of this study is to evaluate the effect of consuming fungicide-treated pollen on larval fitness (measured in terms of development time and prepupal biomass). While exposure to the commonly applied fungicide propiconazole has been linked to increased mortality among adult bees across several species 23,24,32,44,45,54,55,56,57,58,65,66,67, its impact on larval bees is less known. The second objective of this study is to evaluate the effects of consuming non-host pollen on larval fitness. Previous studies indicate that larvae of oligolectic bees fail to develop when forced to consume non-host pollen68. Such results may be attributed to variations in bee physiology69, pollen biochemistry70, and the beneficial microbiome associated with natural pollen provisions71. The third objective of this study is to evaluate the interactive effects of fungicide treatment and dietary pollen on larval fitness.
Numerous biological traits including maternal body size, provisioning rate, foraging strategy, and pollen quantity72,73,74,75 are known to affect larval fitness among solitary bees. These factors can introduce significant variability between reeds, which poses a challenge in developing defensible experimental designs when assessing larval health. Moreover, given that larval development occurs inside sealed nesting reeds, the effects of such variability on the progeny are difficult to visualize and quantified without using non-lethal techniques (Figure 3). To overcome this challenge, all hypotheses within this study are tested using larvae reared outside of their nesting reeds. The experimental design represents a fully crossed 2 × 2 factorial set-ups, with each factor consisting of 2 levels; Factor 1: Fungicide exposure (Fungicide; No fungicide); Factor 2: Pollen source (Host pollen, Non-host pollen). Bees are raised from the egg to the prepupal stage within sterile multiwell cell culture plates under controlled laboratory conditions. Each well is individually stocked with a standardized amount of pollen provision and a single egg. After hatching, the larva feeds on the allocated pollen within the well, completes larval development, and initiates pupation. Past studies have shown that unexplained mortality is lower among bees raised within this artificial rearing environment than that encountered in the wild49,76. The use of in vitro-reared bees has several advantages over field-based studies: 1) it minimizes the confounding effects of natural variability and uncontrolled factors typically associated with field-based studies; 2) it allows multiple levels of manipulation for each factor(s) of interest to be tested simultaneously across treatment groups; 3) the number of replicates can be predetermined, and experimental factors for each replicate can be individually manipulated; 4) larval response variables can be easily visualized and recorded independently without disturbing adjacent larvae; 5) the protocol can be modified to accommodate more complex experimental designs involving multiple factors and response variables.

Figure 3. Contents within a natural nesting reed of Osmia ribifloris (s.l.). Close up of (A) a dissected reed showing individual chambers, pollen provisions, and partitions, and (B) freshly harvested pollen provisions, and the associated eggs (indicated with a black circle). Please click here to view a larger version of this figure.
Protokół
1. Prepare Propiconazole Solutions for Fungicide Exposure Experiments
- Prepare 0.1x fungicide solution by dissolving appropriate volumes of commercially purchased propiconazole 14.3% in sterile water the day of the experiment. Ensure that only freshly prepared fungicide solution is used for all treatments.
- Add 23 µL of 0.1x fungicide solution per gram of pollen provision to obtain the maximum concentration of propiconazole previously reported from bee-collected pollen24 (0.361 PPM or µg of active ingredient g-1 of pollen).
2. Harvest Eggs and Host Pollen Provisions from Osmia Reeds
- Using a sterilized scalpel, dissect freshly plugged nesting reeds of Osmia, splitting it into two parts along the length of the reed to expose the individual chambers.
NOTE: Each nest may contain between 8 to 14 chambers and a single egg within a chamber. - Inspect the reeds visually to identify the chambers containing male eggs based on previously published guidelines77. Use a sterilized bent needle to remove each pollen provision along with the associated egg from the nesting reed, and place in a clean weigh boat.
- Gently separate the egg from the provision using a clean fine paint brush and record the fresh weight of the pollen provision and egg using a standard laboratory balance. Calculate the average weight of the male pollen provisions.
- Perform the subsequent steps with minimum delay to reduce the chances of damage to the egg from exposure to excess temperature and dehydration.
3. Prepare Host Plant Pollen Provisions
- Visually inspect the maternally collected host-plant pollen excavated from the nesting chambers to ensure that no parasites are present78. In order to reduce any potential maternal bias, combine the pollen provisions into a single mass in a sterile petri dish and mix well using a sterilized needle.
- Divide the combined mass into new pollen provisions, ensuring that the weight of each reconstituted provision is approximately equal to the average weight of a naturally allocated male provision (Mean ± SE, 0.35 ± 0.01 g, N = 42).
NOTE: Because Osmia sp. allocates smaller pollen provisions to the male offspring, this results in lower body weights of the male larvae compared to that of females77. To avoid any such bias resulting from sex-specific differences, only use male eggs in the experiments.
4. Prepare Non-Host Plant Pollen Provision
- Pulverize commercially purchased honey bee-collected pollen to a fine powder using a standard laboratory ball-mill.
- Based on the moisture content of maternally-collected host pollen provisions (~20%), hydrate the pollen powder using appropriate volumes of 40% sterilized sugar solution79 and mix thoroughly to form a dough-like consistency.
- Divide into individual pollen masses, each weighing approximately the same as the average weight of a naturally allocated male provision.
NOTE: Moisture content of maternally collected host pollen provisions can be standardized in prior by comparing the fresh and dry weight of pollen provisions from 30 randomly selected male chambers80. To obtain the dry weight, pollen provisions should be freeze-dried in a lyophilizer (1.5 Pa for 72 h).
5. Prepare Multiwell Cell Culture Plates
- Line individual wells of sterile 48-well culture plate with autoclaved tin cups (5 × 9 cm). Using sterile forceps, gently flair out the top rim of the capsule so that it may accommodate the pollen provision.
- Place a single mass of host or non-host pollen provision inside the tin cup using sterile tools based on the treatment group.
NOTE: To avoid cross-contamination, use separate plates for treatment and control groups.
6. Add Fungicides
- Make a centrally placed depression within the pollen mass using a sterile wooden stick. Use a new stick for each pollen provision.
- Add appropriate volumes of fungicide solution (for treatment), or sterile water (for controls) into the depression. Pinch the opening of the depression using sterile forceps to minimize surface contact between the fungicide/ sterile water and the egg.
- Ensure that the factorial set-up of the experimental design aligns with that depicted in the schematic representation (Figure 4).
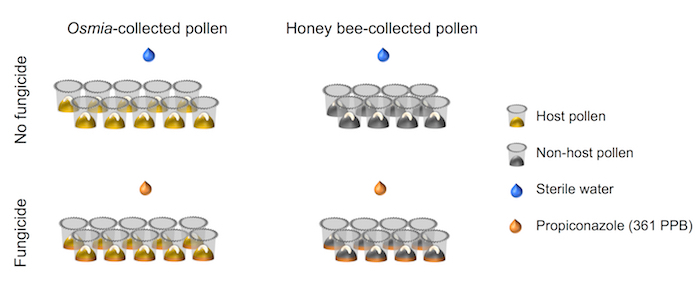
Figure 4. Schematic representation of the experimental setup. The experiment represents a fully-crossed 2 × 2 factorial setups. Factor 1 represents Fungicide exposure and consists of 2 levels: (i) No fungicide (N = 10), and (ii) Fungicide (N = 10). Factor 2 represents Pollen source and consists of 2 levels: (i) Host pollen (N = 8), and (ii) Non-host pollen (N = 8). Please click here to view a larger version of this figure.
7. Rear and Observe Larvae
- Place a randomly selected male egg on the top surface of the pollen provision using a clean fine paint brush. Once eggs have been placed on all the provisions, replace the lid of the cell culture plate, securing it with labeling tape on the corners.
- Place the well plates on a clean tray and cover it with a dark cloth to obstruct contact with direct light. Place a 6 well plate containing 30 mL of sterile water within the tray to prevent desiccation. Leave incubation trays undisturbed inside an incubator at room temperature.
- Observe well plates daily under a dissecting microscope without removing the lid of the well plates. Ensure that the larvae are alive by checking for movement. If no movement is detected, discard the tin cup containing the dead larvae and the remaining pollen provision. Allow all surviving larvae to develop undisturbed within the well plates till they reach the prepupal stage.
- Remove the larva from the tin cup once it reaches the prepupal stage41. Use a brush to clean any defecate from the silk cocoon. Carefully cut through the silk cocoon using a dissecting microscope and extract the prepupa with rubber forceps.
- Handle the prepupa gently to ensure that the tools do not pierce the soft body. Record the fresh weight of the prepupa (prepupal biomass) and the developmental time from egg to the prepupal stage (larval developmental time).
NOTE: Any dead larva should be discarded immediately to prevent undesired microbial growth on the cadaver and leftover pollen provision. This reduces the risk of infection to the remaining healthy larvae.
Wyniki
Larval fitness was quantified using three metrics (i) larval developmental time, (ii) prepupal biomass, and (iii) percent survivorship. A two-way ANOVA was conducted using Fungicide exposure (two levels: No fungicide, Fungicide) and Pollen source (two levels: Host pollen, Non-host pollen) as the independent variables, and larval developmental time as the dependent variable. The main effect for Fungicide exposure (F1,28 = 1.24, P = 0.28) was non-significant bet...
Dyskusje
Rearing bees outside their natural nesting reeds, under laboratory conditions, allows the testing of multiple hypotheses pertaining to larval fitness. To the extent that unidentified factors continue to cause bee mortality, risk assessment studies using in vitro experiments can help identify potential threats and inform management practices for this species-rich group of wild pollinators 12,38,49,
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors thank Kimball Clark and Tim Krogh for providing Osmia nesting reeds, Meredith Nesbitt and Molly Bidwell for assistance in the lab, Drs. Cameron Currie, Christelle Guédot, Terry Griswold, Michael Branstetter and three anonymous reviewers for their useful comments that improved the manuscript. This work was supported by USDA-Agricultural Research Service appropriated funds (Current Research Information System #3655-21220-001), Wisconsin Department of Agriculture, Trade, and Consumer Protection (#197199), National Science Foundation (under Grant No. DEB-1442148), the DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494).
Materiały
| Name | Company | Catalog Number | Comments |
| eggs of O. ribifloris sensu lato (s.l.) | Kaysville, Davis County, Utah, USA | ||
| Osmia reeds | Nativebees.com | NA | Freshly plugged reeds |
| Dissection set | VWR | 89259-964 | Sterilize before use |
| Long Nose Pliers | Husky | 1006 | |
| 6 well culture plates | VWR | 10062-892 | Sterile sealed |
| 48 well culture plates | VWR | 10062-898 | Sterile sealed |
| Petri dishes | VWR | 25373100 | Sterile sealed |
| Square Weighing Boats | VWR | 10770-448 | |
| Camel Hair Brush | Bioquip | 1153A | |
| Tin capsules | EA Consumables | D1021 | Sterilize before use |
| Sucrose | VWR | 470302-808 | |
| Propiconazole 14.3 | Quali-Ppro | 60207-90-1 | Propiconazole 14.3% |
| Honey bee pollen | Bee energised | 897098001244 | Untreated, natural, raw pollen |
| Microbalance | VWR | 10204-990 | |
| Pulverisette | LAB SYNERGY INC. | 30334913 | |
| Wooden sticks | VWR | 470146908 | Sterilize before use |
| Sealing tape | VWR | 89097-912 | |
| Microscope | VWR | 89403-384 | |
| Planting tray | VWR | 470150-632 | |
| Ethanol | VWR | BDH1158-4LP | |
| Centrifuge tube | VWR | 21008936 | |
| Microsyringe | Cole-Palmer | UX-07940-07 | |
| Rubber tweezer | Amazon | B0135HWPN4 | |
| Syringe needles | VWR | 89219-334 | |
| Freeze drier | Labcono | LFZ-1L | |
| Statistical software | SPSS | Version 21.0 |
Odniesienia
- Klein, A. -. M., et al. Importance of pollinators in changing landscapes for world crops. P Roy Soc Lond B Bio. 274 (1608), 303-313 (2007).
- Biesmeijer, J. C. J., et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 313 (5785), 351-354 (2006).
- Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., Kunin, W. E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol Evol. 25 (6), 345-353 (2010).
- Cameron, S. A., et al. Patterns of widespread decline in North American bumble bees. P Natl Acad Sci USA. 108 (2), 662-667 (2011).
- Gallai, N., Salles, J. M., Settele, J., Vaissière, B. E. Economic valuation of the vunerability of world agriculture confronted with pollinator decline. Ecol Econ. 68 (3), 810-821 (2009).
- Fontaine, C., Dajoz, I., Meriguet, J., Loreau, M. Functional diversity of plant-pollinator interaction webs enhances the persistence of plant communities. Plos Biol. 4 (1), 0129-0135 (2006).
- Kluser, S., Peduzzi, P. . Global pollinator decline: a literature review. , (2007).
- Brown, M. J. F., Paxton, R. J. The conservation of bees: a global perspective. Apidologie. 40 (3), (2009).
- Lebuhn, G., et al. Detecting insect pollinator declines on regional and global scales. Conserv Biol. 27 (1), (2013).
- Vanengelsdorp, D., Meixner, M. D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol. , S80-S95 (2010).
- Pettis, J. S., Delaplane, K. S. Coordinated responses to honey bee decline in the USA. Apidologie. 41 (3), 256-263 (2010).
- Sandrock, C., Tanadini, L. G., Pettis, J. S., Biesmeijer, J. C., Potts, S. G., Neumann, P. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agr Forest Entomol. 16 (2), (2014).
- Van der Sluijs, J. P., Simon-Delso, N., Goulson, D., Maxim, L., Bonmatin, J. M., Belzunces, L. P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr Opin Env Sust. 5 (3), (2013).
- Goulson, D., Nicholls, E., Botías, C., Rotheray, E. L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 347 (6229), (2015).
- Johnson, R. M., Ellis, M. D., Mullin, C. A., Frazier, M. Pesticides and honey bee toxicity - USA. Apidologie. 41 (3), (2010).
- Iwasa, T., Motoyama, N., Ambrose, J. T., Roe, R. M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Protection. 23 (5), 371-378 (2004).
- Glavan, G., Bozic, J. The synergy of xenobiotics in honey bee Apis mellifera: mechanisms and effects. Acta Biol. Slov. 56, 11-27 (2013).
- Biddinger, D. J., et al. Comparative toxicities and synergism of apple orchard pesticides to Apis mellifera (L.) and Osmia cornifrons (Radoszkowski). PLoS ONE. 8 (9), e72587 (2013).
- Thompson, H. M., Fryday, S. L., Harkin, S., Milner, S. Potential impacts of synergism in honeybees (Apis mellifera) of exposure to neonicotinoids and sprayed fungicides in crops. Apidologie. 45 (5), 545-553 (2014).
- Jansen, J. -. P., Lauvaux, S., Gruntowy, J., Denayer, J. Possible synergistic effects of fungicide-insecticide mixtures on beneficial arthropods. IOBC-WPRS Bulletin. 125, 28-35 (2017).
- Robinson, A., Hesketh, H., et al. Comparing bee species responses to chemical mixtures: Common response patterns?. PLoS ONE. 12 (6), (2017).
- Sgolastra, F., Medrzycki, P., et al. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Management Science. 73 (6), 1236-1243 (2017).
- Ladurner, E., Bosch, J., Kemp, W. P., Maini, S. Assessing delayed and acute toxicity of five formulated fungicides to Osmia lignaria and Apis mellifera. Apidologie. 36 (3), 449-460 (2005).
- Mullin, C. A., et al. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PloS one. 5 (3), e9754 (2010).
- Pettis, J. S., Lichtenberg, E. M., Andree, M., Stitzinger, J., Rose, R., Vanengelsdorp, D. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PloS one. 8 (7), e70182 (2013).
- David, A., et al. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ Int. 88, 169-178 (2016).
- Zhu, W., Schmehl, D. R., Mullin, C. A., Frazier, J. L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PloS one. 9 (1), e77547 (2014).
- Simon-Delso, N., Martin, G. S., Bruneau, E., Minsart, L. A., Mouret, C., Hautier, L. Honeybee colony disorder in crop areas: The role of pesticides and viruses. PLoS ONE. 9 (7), (2014).
- Park, M. G., Blitzer, E. J., Gibbs, J., Losey, J. E., Danforth, B. N. Negative effects of pesticides on wild bee communities can be buffered by landscape context. P Roy Soc B-Biol Sci. 282 (1809), 20150299-20150299 (2015).
- Bernauer, O. M., Gaines-Day, H. R., Steffan, S. A. Colonies of bumble bees (Bombus impatiens) produce fewer workers, less bee biomass, and have smaller mother queens following fungicide exposure. Insects. 6 (2), 478-488 (2015).
- Williamson, S. M., Wright, G. A. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol. 216 (10), 1799-1807 (2013).
- Artz, D. R., Pitts-Singer, T. L. Effects of fungicide and adjuvant sprays on nesting behavior in two managed solitary bees, Osmia lignaria and Megachile rotundata. PLoS ONE. 10 (8), e0135688 (2015).
- Pilling, E. D., Bromleychallenor, K. A. C., Walker, C. H., Jepson, P. C. Mechanism of synergism between the pyrethroid insecticide lambda-cyhalothrin and the imidazole fungicide prochloraz, in the honeybee (Apis mellifera L). Pestic Biochem Phys. 51 (1), 1-11 (1995).
- Johnson, R. M., Wen, Z., Schuler, M. A., Berenbaum, M. R. Mediation of pyrethroid insecticide toxicity to honey bees (Hymenoptera: Apidae) by cytochrome P450 monooxygenases. J. Econ. Entomol. 99 (4), 1046-1050 (2006).
- Steffan, S. A., Dharampal, P. S., Diaz-Garcia, L. A., Currie, C. R., Zalapa, J. E., Hittinger, C. T. Empirical, metagenomic, and computational techniques illuminate the mechanisms by which fungicides compromise bee health. JoVE. (128), e54631 (2017).
- Batra, S. W. T. Solitary bees. Sci Am. 250 (2), 120-127 (1984).
- Linsley, E. G. The ecology of solitary bees. Hilgardia. 27 (19), 543-599 (1958).
- Garibaldi, L. A., et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science. 339 (6127), 1608-1611 (2013).
- Bosch, J., Kemp, W. P. . How to manage the blue orchard bee. , (2001).
- Keller, A., Grimmer, G., Steffan-Dewenter, I. Diverse microbiota identified in whole intact nest chambers of the red mason bee Osmia bicornis (Linnaeus 1758). PLoS ONE. 8 (10), e78296 (2013).
- Bosch, J., Kemp, W. P. Development and Emergence of the Orchard Pollinator Osmia lignaria (Hymenoptera: Megachilidae). Environmental Entomology. 29 (1), 8-13 (2000).
- Brittain, C., Potts, S. G. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic and Applied Ecology. 12 (4), 321-331 (2011).
- Arena, M., Sgolastra, F. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology. 23 (3), 324-334 (2014).
- Ladurner, E., Bosch, J., Kemp, W. P., Maini, S. Foraging and nesting behavior of Osmia lignaria (Hymenoptera: Megachilidae) in the presence of fungicides: cage studies. J Econ Entomol. 101 (3), 647-653 (2008).
- Huntzinger, A. C. I., James, R. R., Bosch, J., Kemp, W. P. Fungicide tests on adult alfalfa leafcutting bees (Hymenoptera: Megachilidae). J Econ Entomol. 101 (4), 1088-1094 (2008).
- Tsvetkov, N., et al. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science. 356 (6345), 1395-1397 (2017).
- Mao, W., Schuler, M. A., Berenbaum, M. R. Disruption of quercetin metabolism by fungicide affects energy production in honey bees (Apis mellifera). P Natl Acad Sci. 114 (10), 2538-2543 (2017).
- Blacquière, T., Smagghe, G., Van Gestel, C. A. M., Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology. 21 (4), 973-992 (2012).
- Sgolastra, F., Tosi, S., Medrzycki, P., Porrini, C., Burgio, G. Toxicity of spirotetramat on solitary bee larvae, Osmia cornuta (Hymenoptera: Megachilidae), in laboratory conditions. Journal of Apicultural Science. 59 (2), 73-83 (2015).
- Mader, E., Spivak, M., Evans, E. . Managing Alternative Pollinators. , (2010).
- Bosch, J., Kemp, W. P. Developing and establishing bee species as crop pollinators: the example of Osmia spp.(Hymenoptera: Megachilidae) and fruit trees. B Entomol Res. 92 (1), 3-16 (2002).
- Sampson, B. J., Rinehart, T. A., Kirker, G. T., Stringer, S. J., Werle, C. T. Phenotypic variation in fitness traits of a managed solitary bee, Osmia ribifloris (Hymenoptera: Megachilidae). J Econ Entomol. 108 (6), 2589-2598 (2015).
- Sampson, B. J., Cane, J. H., Kirker, G. T., Stringer, S. J., Spiers, J. M. Biology and management potential for three orchard bee species (Hymenoptera: Megachilidae): Osmia ribifloris Cockerell, O. lignaria (Say) and O.chalybea Smith with emphasis on the former. Acta Hort. 810, 549-555 (2009).
- Hladik, M. L., Vandever, M., Smalling, K. L. Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Sci Total Environ. 542, 469-477 (2016).
- Long, E. Y., Krupke, C. H. Non-cultivated plants present a season-long route of pesticide exposure for honey bees. Nat Commun. 7, (2016).
- Krupke, C. H., Hunt, G. J., Eitzer, B. D., Andino, G., Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE. 7 (1), e29268 (2012).
- Stoner, K. A., Eitzer, B. D. Using a hazard quotient to evaluate pesticide residues detected in pollen trapped from honey bees (Apis mellifera) in Connecticut. PLoS ONE. 8 (10), e77550 (2013).
- Sánchez-Bayo, F., Goulson, D., Pennacchio, F., Nazzi, F., Goka, K., Desneux, N. Are bee diseases linked to pesticides? - A brief review. Environ Int. 89, 7-11 (2016).
- Steffan-Dewenter, I., Klein, A. -. M., Gaebele, V., Alfert, T., Tscharntke, T. Bee diversity and plant-pollinator interactions in fragmented landscapes. Specialization and generalization in plant-pollinator interactions. , 387-410 (2006).
- Kremen, C., Ricketts, T. Global perspectives on pollination disruptions. Conserv Biol. 14 (5), 1226-1228 (2000).
- Memmott, J., Waser, N. M., Price, M. V. Tolerance of pollination networks to species extinctions. P Roy Soc B-Biol Sci. 271 (1557), 2605-2611 (2004).
- Spear, D. M., Silverman, S., Forrest, J. R. K. Asteraceae pollen provisions protect Osmia mason bees (Hymenoptera: Megachilidae) from brood parasitism. The American Naturalist. 187 (6), 797-803 (2016).
- Rust, R. W. Biology of Osmia (Osmia) ribifloris Cockerell (Hymenoptera: Megachilidae). J Kansas Entomol Soc. 59, 89-94 (1986).
- Torchio, P. F. Osmia ribifloris, a native bee species developed as a commercially managed pollinator of highbush blueberry (Hymenoptera: Megachilidae). J Kansas Entomol Soc. 63 (633), 427-436 (1990).
- Sanchez-Bayo, F., Goka, K. Pesticide residues and bees - A risk assessment. PLoS ONE. 9 (4), e94482 (2014).
- Kasiotis, K. M., Anagnostopoulos, C., Anastasiadou, P., Machera, K. Pesticide residues in honeybees, honey and bee pollen by LC-MS/MS screening: Reported death incidents in honeybees. Sci Total Environ. 485 (1), 633-642 (2014).
- Stanley, J., Sah, K., Jain, S. K., Bhatt, J. C., Sushil, S. N. Evaluation of pesticide toxicity at their field recommended doses to honeybees, Apis cerana and A. mellifera through laboratory, semi-field and field studies. Chemosphere. 119, 668-674 (2015).
- Praz, C. J., Müller, A., Dorn, S. Specialized bees fail to develop on non-host pollen: Do plants chemically protect their pollen?. Ecology. 89 (3), 795-804 (2008).
- Sedivy, C., Müller, A., Dorn, S. Closely related pollen generalist bees differ in their ability to develop on the same pollen diet: Evidence for physiological adaptations to digest pollen. Funct Ecol. 25 (3), 718-725 (2011).
- Williams, N. M. Use of novel pollen species by specialist and generalist solitary bees (Hymenoptera: Megachilidae). Oecologia. 134, (2003).
- Graystock, P., Rehan, S. M., McFrederick, Q. S. Hunting for healthy microbiomes: determining the core microbiomes of Ceratina, Megalopta, and Apis bees and how they associate with microbes in bee collected pollen. Conserv Genet. 18 (3), 1-11 (2017).
- Bosch, J., Vicens, N. Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behav Ecol Sociobiol. 60 (1), 26-33 (2006).
- Bosch, J., Vicens, N. Body size as an estimator of production costs in a solitary bee. Ecol Entomol. 27 (2), 129-137 (2002).
- Radmacher, S., Strohm, E. Factors affecting offspring body size in the solitary bee Osmia bicornis (Hymenoptera, Megachilidae). Apidologie. 41 (2), 169-177 (2010).
- Seidelmann, K. Open-cell parasitism shapes maternal investment patterns in the Red Mason bee Osmia rufa. Behav Ecol. 17 (5), (2006).
- Becker, M. C., Keller, A. Laboratory rearing of solitary bees and wasps. Insect Science. 23 (6), 918-923 (2016).
- Bosch, J. The nesting behaviour of the mason bee Osmia cornuta (Latr) with special reference to its pollinating potential (Hymenoptera, Megachilidae). Apidologie. 25, 84-93 (1994).
- Krunić, M., Stanisavljević, L., Pinzauti, M., Felicioli, A. The accompanying fauna of Osmia cornuta and Osmia rufa and effective measures of protection. B Insectol. 58 (2), 141-152 (2005).
- Elliott, S. E., Irwin, R. E., Adler, L. S., Williams, N. M. The nectar alkaloid, gelsemine, does not affect offspring performance of a native solitary bee, Osmia lignaria (Megachilidae). Ecol Entomol. 33 (2), 298-304 (2008).
- Hendriksma, H. P., Härtel, S., Steffan-Dewenter, I. Honey bee risk assessment: New approaches for in vitro larvae rearing and data analyses. Methods Ecol and Evol. 2 (5), 509-517 (2011).
- Aupinel, P., et al. Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. B Insectol. 58 (2), 107-111 (2005).
- Beekman, M., Ratnieks, F. L. W. Long-range foraging by the honey-bee, Apis mellifera L. Funct Ecol. 14 (4), 490-496 (2000).
- Gathmann, A., Tscharntke, T. Foraging ranges of solitary bees. J Anim Ecol. 71 (5), 757-764 (2002).
- Greenleaf, S. S., Williams, N. M., Winfree, R., Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia. 153 (3), 589-596 (2007).
- . Bee Pollen Supplement - Bee Rescued Available from: https://beerescued.com/product/bee-rescued-bee-pollen-supplement/ (2018)
- Cane, J. H., Griswold, T., Parker, F. D. Substrates and Materials Used for Nesting by North American Osmia Bees (Hymenoptera: Apiformes: Megachilidae). Annals of the Entomological Society of America. 100 (3), 350-358 (2007).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone