Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Tuning the Acidity of Pt/ CNTs Catalysts for Hydrodeoxygenation of Diphenyl Ether
W tym Artykule
Podsumowanie
A protocol for the synthesis of HNbWO6, HNbMoO6, HTaWO6 solid acid nanosheet modified Pt/CNTs is presented.
Streszczenie
We herein present a method for the synthesis of HNbWO6, HNbMoO6, HTaWO6 solid acid nanosheet modified Pt/CNTs. By varying the weight of various solid acid nanosheets, a series of Pt/xHMNO6/CNTs with different solid acid compositions (x = 5, 20 wt%; M = Nb, Ta; N = Mo, W) have been prepared by carbon nanotube pretreatment, protonic exchange, solid acid exfoliation, aggregation and finally Pt particles impregnation. The Pt/xHMNO6/CNTs are characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy and NH3-temperature programmed desorption. The study revealed that HNbWO6 nanosheets were attached on CNTs, with some edges of the nanosheets being bent in shape. The acid strength of the supported Pt catalysts increases in the following order: Pt/CNTs < Pt/5HNbWO6/CNTs < Pt/20HNbMoO6/CNTs < Pt/20HNbWO6/CNTs < Pt/20HTaWO6/CNTs. In addition, the catalytic hydroconversion of lignin-derived model compound: diphenyl ether using the synthesized Pt/20HNbWO6 catalyst has been investigated.
Wprowadzenie
Many industrial processes for the manufacture of chemicals involve the use of aqueous inorganic acid. One typical example is the conventional H2SO4 process for the hydration of cyclohexane to produce cyclohexanol. The process involves a biphasic system, with the cyclohexane being in the organic phase and the cyclohexanol product being in the acidic aqueous phase, thus making the separation process by simple distillation difficult. Apart from difficulty in separation and recovery, inorganic acid is also highly toxic and corrosive to equipment. Sometimes, the use of inorganic acid generates byproducts that will lower the product yield and must be avoided. For example, the dehydration of 2-cyclohexene-1-ol to produce 1,3-cyclohexadiene using H2SO4 will lead to polymerization byproducts1. Thus, many industrial processes shift towards using solid acid catalysts. Various water tolerant solid acids are used to solve the above problem and to maximize the product yields, such as the use of HZSM-5 and Amberlyst-15. The use of high-silica HZSM-5 zeolite has been shown to replace H2SO4 in the production of cyclohexanol from benzene2. Since the zeolite is present in the neutral aqueous phase, the product will go to the organic phase exclusively, thus simplifying the separation process. However, due to Lewis acid-base adduct formation of water molecules to the Lewis acid sites, zeolitic materials still demonstrated lower selectivity due to the presence of inactive sites3. Among all these solid acids, Nb2O5 is one of the best candidates that contain both Lewis and BrØnsted acid sites. The acidity of Nb2O5∙nH2O is equivalent to a 70% H2SO4 solution, due to the presence of the labile protons. The BrØnsted acidity, which is comparable to protonic zeolite materials, are very high. This acidity will turn to Lewis acidity following water elimination. In the presence of water, Nb2O5 forms the tetrahedral NbO4-H2O adducts, which may decrease in Lewis acidity. However, the Lewis acid sites are still effective since the NbO4 tetrahedral still have effective positive charges4. Such phenomenon has been demonstrated successfully in the conversion of glucose into 5-(hydroxymethyl)furfural (HMF) and the allylation of benzaldehyde with tetraallyl tin in water5. Water-tolerant catalysts are thus crucial in biomass conversion in renewable energy applications, especially when the conversions are performed in environmental benign solvents such as water.
Among the many environmental benign solid acid catalysts, functionalized carbon nanomaterials using graphene, carbon nanotubes, carbon nanofibers, mesoporous carbon materials have been playing an important role in the valorization of biomass due to the tunable porosity, extremely high specific surface area, and excellent hydrophobicity6,7. The sulfonated derivatives are particularly stable and highly active protonic catalytic materials. They can either be prepared by incomplete carbonization of sulfonated aromatic compounds8 or by sulfonation of incompletely carbonized sugars9. They have proven to be very efficient catalysts (e.g., for the esterification of higher fatty acids) with activity comparable to the use of liquid H2SO4. Graphenes and CNTs are carbon materials with a large surface area, excellent mechanical properties, good acid resistance, uniform pore size distributions, as well as resistance to coke deposition. Sulfonated graphene has been found to efficiently catalyze the hydrolysis of ethyl acetate10 and bifunctional graphene catalysts has been found to facilitate the one-pot conversion of levullinic acid to γ-valerolactone11. Bifunctional metals supported on CNTs are also very efficient catalysts for application in biomass conversion12,13 such as the highly selective aerobic oxidation of HMF to 2,5-diformylfuran over the VO2-PANI/CNT catalyst14.
Taking advantage of the unique properties of Nb2O5 solid acid, functionalized CNTs and bifunctional metal supported on CNTs, we report the protocol for the synthesis of a series of Nb(Ta)-based solid acid nanosheet modified Pt/CNTs with a high surface area by a nanosheet aggregation method. Furthermore, we demonstrated that Pt/20HNbWO6/CNTs, as a result of the synergistic effect of well-dispersed Pt particles and strong acid sites derived from HNbWO6 nanosheets, exhibit the best activity and selectivity in converting lignin-derived model compounds into fuels by hydrodeoxygenation.
Protokół
CAUTION: For the proper handling methods, properties and toxicities of the chemicals described in this paper, refer to the relevant material safety data sheets (MSDS). Some of the chemicals used are toxic and carcinogenic and special care must be taken. Nanomaterials may potentially pose safety hazards and health effects. Inhalation and skin contact should be avoided. Safety precautions must be exercised, such as performing the catalyst synthesis in the fume hood and catalyst performance evaluation with autoclave reactors. Personal protective equipment must be worn.
1. Pretreatment of CNTs13
- Immerse 1.0 g of CNTs into 50 mL of nitric acid in a 100 mL beaker.
- Sonicate the solution at 25 °C for 1.5 h to remove surface impurities and to enhance the anchoring effect of the catalyst.
- Transfer the solution into a 100 mL round bottom flask.
- Reflux the solution in a mixture of nitric acid (65%) and sulfuric acid (98%) at 60 °C for overnight. Set the volume ratio at 3:1. This will create surface defects on the CNTs.
- Filter the solution to obtain the multiwall carbon nanotube solid. Wash the solid with deionized water.
- Dry the solid at 80 °C for 14 h.
2. Preparation of HNbWO6 solid acid nanosheets15 by protonic exchange followed by exfoliation
- Mix stoichiometric amounts of Li2CO3 (0.9236 g) and metal oxides Nb2O5 (3.3223 g) and WO3 (5.7963 g) at a molar ratio of 1:1:2.
- Calcine the solid mixture at 800 °C for 24 h with one intermediate grinding.
- Place 10.0 g of LiNbWO6 powder in 200 mL of 2 M HNO3 aqueous solution at 50 °C and stir the solution mixture for 5 days (120 h) with one replacement of the acid at 60 h.
- Exchange the acid liquid every day and repeat step 2.3.
- Filter the solid and wash the solid with deionized water 3x.
- Dry the solid at 80 °C overnight.
- Add an amount of 25 wt.% TBAOH (tetra (n-butylammonium) hydroxide) solution to 150 mL of deionized water solution with 2.0 g of protonated compound obtained in step 2.6 until the pH reaches 9.5 – 10.0.
- Stir the above solution for 7 days.
- Centrifuge the above solution and collect the supernatant solution that contains the dispersed nanosheets.
3. Preparation of HNbMO6 solid acid nanosheets
NOTE: The procedure is similar to that of step 2 except for the first and third steps.
- Mix stoichiometric amounts of Li2CO3 and metal oxides Nb2O5 and MoO3 in a molar ratio of 1:1:2.
- Calcine the above solid mixtures at 800 °C in air for 24 h with one intermediate grinding.
- Place 10.0 g of LiNbMoO6 powder in 200 mL of 2 M HNO3 aqueous solution at 50 °C and stir the solution mixture for 5 days (120 h) with one replacement of the acid at 60 h.
4. Preparation of HTaWO6 solid acid nanosheets
NOTE: The procedure is similar to that of step 2 except for the first and third steps.
- Mix stoichiometric amounts of Li2CO3 and metal oxides Ta2O5 and WO3 in a molar ratio of 1:1:2.
- Calcine the above solid mixtures at 900 °C in air for 24 h with one intermediate grinding.
- Place 10.0 g of LiTaWO6 powder in 200 mL of 2 M HNO3 aqueous solution at 50 °C and stir the solution mixture for 5 days (120 h) with one replacement of the acid at 60 h.
5. Preparation of HNbWO6/MWCNTs by the nanosheet aggregation method
- Add 2.0 g of multiwall CNTs obtained in step 1 to a 100 mL solution of HNbWO6 nanosheets in a 250 mL round bottom flask.
- Add 100 mL of 1.0 M HNO3 aqueous solution into the round bottom flask dropwise. This will aggregate the nanosheets samples.
- Continue to stir the solution at 50 °C for 6 h.
- Filter the solid and wash the solid with deionized water 3x.
- Dry the solid at 80 °C overnight.
- Weigh the dried solid and record the % loading of the solid acid on the MWCNT.
6. Preparation of Pt/20HNbWO6/CNTs by the impregnation method
- Dissolve the H2PtCl6∙H2O into water (1.0 g/100 mL).
- Impregnate the as-prepared nanosheets modified CNTs materials with 1.34 mL of the above Pt aqueous solution.
- Dry the nanosheets CNTs materials at 80 °C, and calcinate the materials at 400 °C for 3 h.
- Obtain the Nb(Ta)-based solid acid nanosheets modified Pt/CNTs catalysts.
7. Hydrodeoxygenation of lignin-derived aromatic ether
NOTE: The chosen lignin-derived aromatic ether is diphenyl ether in this experiment. The chosen lignin-derived aromatic ether is diphenyl ether in this experiment. The activity of Pt/20HTaWO6/CNTs (88.8% conversion, not shown in this paper) is lower than Pt/20HNbWO6/CNTs (99.6%), thus the yield of cyclohexane decreases. Hence, although, higher selectivity of cyclohexane was obtained over Pt/20HTaWO6/CNTs, lower conversion of diphenyl ether limits its utilization. Using appropriate protective equipment and fume hood to perform the reaction using carcinogenic reagents.
- Dilute 0.05 grams of catalyst in 5 milliliters of quartz sand. Load the solution in the middle of a fixed bed reactor between two pillows of quartz wool.
- Reduce the catalyst in H2 (40 mL/min) at 300 °C for 2 h.
- Pump the diphenyl ether feedstocks (including 5.0 wt.% reactant in n-decane and 2.0 wt.% n-dodecane as an internal standard for gas chromatography analysis) into the fixed bed reactor at different flow rates (0.05-0.06 mL/min)
- Collect the products at different space times defined as the ratio between the mass of catalyst W (g) and the flow rate of the substrate F (g/min).
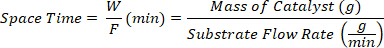
- Identify the liquid products by an GC (HP-5, 30 m x 0.32 mm x 0.25 μm) with 5977A MSD and analyze off-line by gas chromatography (GC 450, FID, FFAP capillary column 30 m x 0.32 mm x 0.25 μm).
- Determine the conversion of reactants (conv.%), selectivity towards product I (Si %), and yield of product i (Yi %) using the following equations:
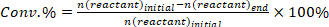 (1)
(1)
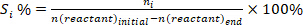 (2)
(2)
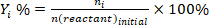 (3)
(3)
Wyniki
X-ray diffraction patterns (XRD) have been studied for the precursor LiNbWO6 and the corresponding proton-exchanged catalyst sample HNbWO6 to determine the phase (Figure 1 and Figure 2). NH3-temperature programmed desorption (NH3-TPD) was used to probe the surface acidity of the catalyst samples (Figure 3). Scanning electron microscopy (SEM) with X-ray microanalysis and...
Dyskusje
Pretreatment of CNTs with nitric acid does increase the specific surface area (SBET) significantly. Raw CNTs have a specific surface area of 103 m2/g while after treatment, the surface area was increased to 134 m2/g. Therefore, such pretreatment to create defects on the CNT surface will have a positive effect on the specific surface area on the catalysts after solid acid modification and platinum particle impregnation. Since the surface area will decrease after incorporation of nanosheets...
Ujawnienia
We have nothing to disclose.
Podziękowania
The work described in this paper was fully supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (UGC/FDS25/E09/17). We also gratefully acknowledge the National Natural Science Foundation of China (21373038 and 21403026) for providing analytical instruments for catalyst characterization and fixed bed reactor for catalyst performance evaluation. Dr. Hongxu Qi would like to thank for the Research Assistantship granted by the Research Grants Council of Hong Kong (UGC/FDS25/E09/17).
Materiały
| Name | Company | Catalog Number | Comments |
| Carbon nanotubes (multi-walled) | Sigma Aldrich | 724769 | |
| Nitric acid (65%) | Sigma Aldrich | V000191 | |
| sulphuric acid (98%) | MERCK | 100748 | |
| Lithium carbonate (>99%) | Aladdin | L196236 | |
| Niobium pentaoxide (99.95%) | Aladdin | N108413 | |
| Tungsten trioxide (99.8%) | Aladdin | T103857 | |
| Molybdenum trioxide (99.5%) | Aladdin | M104355 | |
| Tantalum oxide (99.5%) | Aladdin | T104746 | |
| Chloroplatinic acid hexahydrate, ≥37.50% Pt basis | Sigma Aldrich | 206083 | |
| tetra (n-butylammonium) hydroxide 30-hydrate | Aladdin | D117227 | |
| Diphenyl ether, 98% | Aladdin | D110644 | |
| 2-Bromoacetophenone,98% | Aladdin | B103328 | |
| Diethyl ether,99.5% | Sinopharm | 10009318 | |
| n-Decane,98% | Aladdin | D105231 | |
| n-Dodecane,99% | Aladdin | D119697 | |
| Autoclave Reactor | CJF-0.05—0.1L (Dalian Tongda Equipment Technology Development Co., Ltd) | ||
| Tube furnace | SK2-1-10/12 (Luoyang Huaxulier Electric Stove Co., Ltd) |
Odniesienia
- Jensen, J. L., Uaprasert, V., Fujii, C. R. Acid-Catalyzed Hydration of Dienes. 2. Changes in Activity Coefficient Ratios, Enthalpy, and Entropy as a Function of Sulfuric Acid Concentration. Journal of Organic Chemistry. 41 (10), 1675-1680 (1976).
- Ishida, H., Ono, M., Kaji, S., Watanabe, A. Synthesis of 1,3-Cyclohexadiene through Liquid Phase Dehydration of 2-Cyclohexen-1-ol in Aqueous Solution using Zeolite Catalyst. Nippon Kagaku Kaishi. 4, 267-275 (1997).
- Ishida, H. Liquid-phase hydration process of cyclohexene with zeolites. Catalysis Surveys from Japan. , 241-246 (1997).
- Ushikubo, T., Iizuka, T., Hattori, H., Tanabe, K. Preparation of highly acidic hydrated niobium oxide. Catalysis Today. 16, 291-295 (1993).
- Nakajima, K., et al. Nb2O5.nH2O as a heterogeneous catalyst with water-tolerant Lewis acid sites. Journal of the American Chemical Society. 133 (12), 4224-4227 (2011).
- Lam, E., Luong, J. H. T. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catalysis. 4 (10), 3393-3410 (2014).
- Sudarsanam, P., et al. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chemical Soceity Review. 47 (22), 8349-8402 (2018).
- Hara, M., et al. A carbon material as a strong protonic acid. Angewandte Chemie International Edition English. 43 (22), 2955-2958 (2004).
- Toda, M., et al. Biodiesel made with sugar catalyst. Nature. 438 (7065), (2005).
- Ji, J., et al. Sulfonated graphene as water-tolerant solid acid catalyst. Chemical Science. 2 (3), 484-487 (2011).
- Wang, Y., et al. Graphene-Based Metal/Acid Bifunctional Catalyst for the Conversion of Levulinic Acid to γ-Valerolactone. ACS Sustainable Chemistry & Engineering. 5 (2), 1538-1548 (2016).
- Ma, Q., et al. Catalytic depolymerization of lignin for liquefied fuel at mild condition by rare earth metals loading on CNT. Fuel Processing Technology. , 220-225 (2017).
- Rahzani, B., Saidi, M., Rahimpour, H. R., Gates, B. C., Rahimpour, M. R. Experimental investigation of upgrading of lignin-derived bio-oil component anisole catalyzed by carbon nanotube-supported molybdenum. RSC Advances. 7 (17), 10545-10556 (2017).
- Guo, Y., Chen, J. Bicomponent Assembly of VO2and Polyaniline-Functionalized Carbon Nanotubes for the Selective Oxidation of Biomass-Based 5-Hydroxymethylfurfural to 2,5-Diformylfuran. ChemPlusChem. 80 (12), 1760-1768 (2015).
- He, J., et al. Characterization of HNbMoO6, HNbWO6 and HTiNbO5 as solid acids and their catalytic properties for esterification reaction. Applied Catalysis A: General. , 145-152 (2012).
- Tagusagawa, C., Takagaki, A., Hayashi, S., Domen, K. Characterization of HNbWO6 and HTaWO6 Metal Oxide Nanosheet Aggregates As Solid Acid Catalysts. Journal of Physical Chemistry C. 113, 7831-7837 (2009).
- Niwa, M., Katada, N., Sawa, M., Murakami, Y. Temperature-Programmed Desorption of Ammonia with Readsorption Based on the Derived Theoretical Equation. Journal of Physical Chemistry. 99, 8812-8816 (1995).
- Leiva, K., et al. Conversion of guaiacol over supported ReOx catalysts: Support and metal loading effect. Catalysis Today. , 228-238 (2017).
- Deng, W., Liu, M., Tan, X., Zhang, Q., Wang, Y. Conversion of cellobiose into sorbitol in neutral water medium over carbon nanotube-supported ruthenium catalysts. Journal of Catalysis. 271 (1), 22-32 (2010).
- Huang, B., Huang, R., Jin, D., Ye, D. Low temperature SCR of NO with NH3 over carbon nanotubes supported vanadium oxides. Catalysis Today. 126 (3-4), 279-283 (2007).
- Takagaki, A., Tagusagawa, C., Hayashi, S., Hara, M., Domen, K. Nanosheets as highly active solid acid catalysts for green chemical syntheses. Energy & Environmental Science. 3 (1), 82-93 (2010).
- Hu, L. -. F., et al. Structure and photocatalytic performance of layered HNbWO6nanosheet aggregation. Journal of Nanophotonics. 9 (1), (2015).
- Geim, A. K. Graphene: Status and Prospects. Science. 324, 1530-1534 (2009).
- Golberg, D., et al. Boron Nitride Nanotubes and Nanosheets. ACS Nano. 4 (6), 2979-2993 (2010).
- Wilson, J. A., Yoffe, A. D. The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties. Advances in Physics. 18 (73), 193-335 (1969).
- Ma, R., Sasaki, T. Nanosheets of oxides and hydroxides: Ultimate 2D charge-bearing functional crystallites. Advanced Materials. 22 (45), 5082-5104 (2010).
- Pope, T. R., Lassig, M. N., Neher, G., Weimar Iii, R. D., Salguero, T. T. Chromism of Bi2WO6 in single crystal and nanosheet forms. Journal of Materials Chemistry C. 2 (17), 3223-3230 (2014).
- Yu, Y., et al. Controlled scalable synthesis of uniform, high-quality monolayer and few-layer MoS2 films. Scientific Reports. 3, 1866 (2013).
- Prasomsri, T., Shetty, M., Murugappan, K., Román-Leshkov, Y. Insights into the catalytic activity and surface modification of MoO3 during the hydrodeoxygenation of lignin-derived model compounds into aromatic hydrocarbons under low hydrogen pressures. Energy & Environmental Science. 7 (8), 2660-2669 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone