Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Determination of Chemical Inhibitor Efficiency against Intracellular Toxoplasma Gondii Growth Using a Luciferase-Based Growth Assay
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Presented here is a protocol to evaluate the inhibition efficacy of chemical compounds against in vitro intracellular growth of Toxoplasma gondii using a luciferase-based growth assay. The technique is used to confirm inhibition specificity by genetic deletion of the corresponding target gene. The inhibition of LHVS against TgCPL protease is evaluated as an example.
Streszczenie
Toxoplasma gondii is a protozoan pathogen that widely affects the human population. The current antibiotics used for treating clinical toxoplasmosis are limited. In addition, they exhibit adverse side effects in certain groups of people. Therefore, discovery of novel therapeutics for clinical toxoplasmosis is imperative. The first step of novel antibiotic development is to identify chemical compounds showing high efficacy in inhibition of parasite growth using a high throughput screening strategy. As an obligate intracellular pathogen, Toxoplasma can only replicate within host cells, which prohibits the use of optical absorbance measurements as a quick indicator of growth. Presented here is a detailed protocol for a luciferase-based growth assay. As an example, this method is used to calculate the doubling time of wild-type Toxoplasma parasites and measure the efficacy of morpholinurea-leucyl-homophenyl-vinyl sulfone phenyl (LHVS, a cysteine protease-targeting compound) regarding inhibition of parasite intracellular growth. Also described, is a CRISPR-Cas9-based gene deletion protocol in Toxoplasma using 50 bp homologous regions for homology-dependent recombination (HDR). By quantifying the inhibition efficacies of LHVS in wild-type and TgCPL (Toxoplasma cathepsin L-like protease)-deficient parasites, it is shown that LHVS inhibits wild-type parasite growth more efficiently than Δcpl growth, suggesting that TgCPL is a target that LHVS binds to in Toxoplasma. The high sensitivity and easy operation of this luciferase-based growth assay make it suitable for monitoring Toxoplasma proliferation and evaluating drug efficacy in a high throughput manner.
Wprowadzenie
Toxoplasma gondii is a highly successful obligate intracellular parasite that infects approximately one-third of the human population. Its high transmission rate is predominantly due to its diverse routes of transmission, including consumption of undercooked meat, exposure to mammalian reservoirs, and congenital transmission during birth. T. gondii mainly causes opportunistic infections that can lead to severe morbidity and mortality in immunocompromised individuals1,2,3,4,5,6. The antibiotics currently used for treating acute toxoplasmosis are particularly inefficient in treating congenital and latent infections and cause severe reactions in some individuals3,7,8. Thus, an urgent need to identify novel therapeutics exists. Understanding the differences in subcellular processes within Toxoplasma and its host will help to identify potential drug targets. Therefore, efficient and convenient genome manipulation techniques are required to study the roles of individual genes within Toxoplasma. Additionally, Toxoplasma belongs to the phylum Apicomplexa, which includes several other significant human pathogens, such as Plasmodium spp. and Cryptosporidium spp. Hence, Toxoplasma can be used as a model organism to help study basic biology in other apicomplexan parasites.
To identify novel antibiotics against microbial pathogens, high throughput screening of a library of chemical compounds is initially performed to determine their efficacy in the repression of microbial growth. So far, several microplate-based growth assays have been developed for measuring intracellular growth of T. gondii (i.e., radioactive 3H-uracil incorporation-based quantification9, quantitative ELISA-based parasite detection using T. gondii-specific antibodies10,11, reporter protein-based measurement using β-galactosidase or YFP-expressing Toxoplasma strains12,13, and a recently developed high-content imaging assay14).
These individual strategies all have unique advantages; however, certain limitations also restrict their applications. For example, since Toxoplasma can only replicate within nucleated animal cells, autofluorescence and non-specific binding of anti-T. gondii antibodies to host cells cause interference in fluorescence-based measurements. Furthermore, usage of radioactive isotopes requires special safety compliance and potential safety issues. Some of these assays are more suitable for assessing growth at a single timepoint rather than continuous monitoring of growth.
Presented here is a luciferase-based protocol for the quantification of intracellular Toxoplasma growth. In a previous study, the NanoLuc luciferase gene was cloned under the Toxoplasma tubulin promoter, and this luciferase expression construct was transfected into wild-type (RHΔku80Δhxg strain) parasites to create an RHΔku80Δhxg::NLuc strain (referred to as RHΔku80::NLuc hereafter)15. This strain served as the parental strain for intracellular growth determination and gene deletion in this study. Using the RHΔku80::NLuc strain, parasite growth in human foreskin fibroblasts (HFFs) was monitored over a 96 h period post-infection to calculate parasite doubling time.
In addition, the inhibition efficacy of LHVS against parasite growth can be determined by plotting Toxoplasma growth rates against serial LHVS concentrations to identify the IC50 value. Previous literature has reported that TgCPL is a major target of LHVS in parasites and that treatment with LHVS decreases the development of acute and chronic Toxoplasma infections16,17,18,19. Additionally, RHΔku80::NLuc was used as the parental strain for genome modification to generate a TgCPL-deficient strain (RHΔku80Δcpl::NLuc), and the inhibition of LHVS was measured against this mutant. By observing an upshift of IC50 values for LHVS in the TgCPL-deficient parasites compared to the WT strain, it was validated that TgCPL is targeted by LHVS in vivo.
In this protocol, RHΔku80::NLuc is used as the parental strain, which lacks an efficient non-homologous end-joining pathway (NHEJ), thereby facilitating double crossover homology-dependent recombination (HDR)20,21. Additionally, 50 bp homologous regions are flanked at both ends of a drug resistance cassette by PCR. The PCR product serves as a repair template to remove the entire gene locus via HDR using CRISPR-Cas9-based genome editing tools. Such short homologous regions can be easily incorporated into primers, providing a convenient strategy for production of the repair template. This protocol can be modified to perform universal gene deletion and endogenous gene tagging.
For instance, in our most recent publication, three protease genes, TgCPL, TgCPB (Toxoplasma cathepsin B-like protease), and TgSUB1 (Toxoplasma subtilisin-like protease 1), were genetically ablated in TgCRT (Toxoplasma chloroquine-resistance transporter)-deficient parasites using this method15. Additionally, TgAMN (a putative aminopeptidase N [TgAMN, TGGT1_221310]) was endogenously tagged15. The Lourido lab also reported using short homologous regions in the range of 40-43 bp for the introduction of site-directed gene mutation and endogenous gene tagging in the Toxoplasma genome using a similar method22. These successful genome modifications suggest that a 40-50 bp homologous region is sufficient for efficient DNA recombination in the TgKU80-deficient strain, which greatly simplifies genome manipulation in Toxoplasma gondii.
Protokół
Toxoplasma gondii is categorized in Risk Group 2 and must be handled at a Biosafety Level 2 (BSL-2). The protocol has been reviewed and approved by the Institutional Biosafety Committee at Clemson University.
1. Luciferase-based Toxoplasma growth assay
- Seed human foreskin fibroblasts (HFFs) 1 week before parasite inoculation to ensure that host cells are fully confluent. Perform a mock assay in a transparent plate to ensure that parasites remain intracellular throughout the evaluation period.
NOTE: Here, the assay is conducted in 96 well microplates. According to experimental needs, it can be scaled up to 384 or 1536 well microplates. - Pass Toxoplasma parasites into confluent HFFs 2 days prior to use by transferring ~0.3-0.4 mL of fully lysed parasites into a T25 flask. Incubate infected host cells at 37 °C with 5% CO2 for 2 days.
- Syringe 5 mL of freshly lysed parasites through a 21 G safety needle 5x to liberate intracellular parasites, then pass through a 3 µm filter to remove host cell debris. Rinse residual parasites out of the flask using 7 mL of phenol red-free D10 medium, then pass through the filter again.
- Centrifuge parasites at 1000 x g for 10 min at room temperature (RT). Pour off the supernatant and resuspend the pellet in 10 mL of phenol red-free D10 media.
- Count parasites using a hemocytometer to determine the concentration.
- Dilute parasites to 1 x 104 parasites/mL for the wild-type (WT) strain. For growth-deficient parasite strains, increase the concentration accordingly to observe a significant increase in luciferase signals.
- Aspirate media carefully from 96 well microplates pre-seeded with HFFs and inoculate 150 µL of parasite resuspension into wells in a format of three columns and five rows, which represents three technical replicates and five timepoints.
- Incubate the microplate at 37 °C and 5% CO2 for 4 h.
- Aspirate media carefully from the wells to remove non-invaded parasites, then fill the wells with RT phenol red-free media in each row (except for the first row).
- Mix equal volumes of PBS and 2x luciferase assay buffer and dilute the luciferase substrate to 12.5 µM.
- Add 100 µL of dilute luciferase substrate into each well of the top row. Incubate the microplates at RT for 10 min to allow the cells to fully lyse.
- Measure the luciferase activity using a microplate reader. The plate reader settings are listed in Table 1. Each reading represents the initial number of invaded parasites at 4 h post-infection.
- Repeat steps 1.9-1.12 for each row every 24 h for 4 days without changing the medium. These readings reflect the total number of replicated parasites at 24 h, 48 h, 72 h, and 96 h post-infection.
- Calculate the average readings at each timepoint and divide them by the average readings at 4 h to determine the fold changes in parasite growth over time.
- Plot the data using graphing software. A representative growth reading table and plots of RHΔku80::NLuc parasites are shown in Figure 1A,B.
- To calculate doubling time, plot the log2 values of fold changes at the individual timepoints over the incubation time. Use a linear regression function to calculate slope, which represents the doubling time of each strain (Figure 1A,C).
2. Evaluation of chemical compound inhibition efficacy against Toxoplasma growth
NOTE: Here, evaluation of the inhibition of LHVS in Toxoplasma growth is presented as an example. Eight different concentrations of LHVS are tested, and three technical replicates are performed for each of the three biological replicates for both RHΔku80::NLuc and RHΔku80Δcpl::NLuc strains.
- Prior to the parasite infection, seed HFFs to 96 well microplates in the format of three rows and nine columns for one biological replicate per compound per strain. Host cells will be allowed to grow for at least 7 days before use.
- Pass RHΔku80::NLuc and RHΔku80Δcpl::NLuc parasites for 2 days prior to use. Follow steps 1.2-1.6 for parasite purification and quantification. Resuspend parasites in phenol red-free media at 1 x 104 parasites/mL.
- Aspirate media from a plate containing confluent HFFs and inoculate each well with 150 µL of parasite resuspension. Incubate the microplate at 37 °C and 5% CO2 for 4 h.
- Prepare LHVS at eight different concentrations in a 12 well reservoir by serial dilution. Generally, the concentrations are decreased by three-fold in a serial dilution manner.
NOTE: The lowest concentration is reduced by 6,561-fold relative to the highest concentration. The fold change of the dilution can be adjusted accordingly based on different properties of individual compounds. - At 4 h post-infection, aspirate media to remove non-invaded parasites and fill each well from columns 2-9 with 150 µL of media supplemented with LHVS at different concentrations. Leave the first column filled with regular medium to serve as a nontreated control.
- Incubate the microplate at 37 °C and 5% CO2 for an additional 96 h.
- Perform steps 1.9-1.11 and measure luciferase activity of individual wells.
- Average the luciferase activities of three technical replicates from wells of each individual LHVS concentration.
- Divide the average luciferase activity for each LHVS concentration by the average luciferase activity derived from nontreated parasites to calculate the normalized luciferase activity as a percentage.
- Plot the normalized luciferase activities against the individual LHVS concentrations using graphing software (Figure 2). Inhibition of pyrimethamine against parasite growth is also measured as a control. Pyrimethamine is a clinical antibiotic used to treat acute toxoplasmosis by inhibiting folic acid metabolism in Toxoplasma.
- Calculate the IC50 values for individual compounds using the embedded method in the graphing software, normalized response vs. [inhibitor], under the "dose-response-inhibition" regression program. The IC50 is calculated using the following formula:
Y = 100/(1 + X/IC50)
Where: Y represents normalized luciferase activities of infected cells under different concentrations of inhibitor, and X represents individual concentrations of inhibitor.
3. CRISPR-Cas9-based gene deletion in Toxoplasma parasites
- Generation of a plasmid construct expressing guide RNA (sgRNA) and Cas9 for deleting a gene of interest
- Go to www.ToxoDB.org and retrieve the entire gene coding sequence, including introns and exons, along with 1.5 kb 5'-UTRs and 3'-UTRs (untranslated regions).
NOTE: Here, TgCPL (TGGT1_321530) is targeted as a representative example. - Copy the retrieved TgCPL sequence into the sequence analysis software (refer to Table of Materials for the name and version) and label the 5'- and 3'-UTR regions.
- Select the Tools icon in the top menu bar, then select Cloning | Find CRISPR Sites.
- Choose 3'(Cas9)' for the PAM site location and select the folder containing the Toxoplasma genome sequence in the specificity scoring section. Leave the rest of the settings as defaults.
- Choose a sgRNA with the following two criteria: 1) showing a high specificity score, generally >98%, and 2) lacking a G following the NGG, a protospacer adjacent motif (PAM) sequence. The selected sgRNA is usually located at sites close to the start and stop codons of the gene of interest.
- Copy the sequence of the selected sgRNA and paste it into the following primer template.

The portion in red represents the selected TgCPL sgRNA sequence. It can be replaced with different sgRNAs for various genes of interest.
NOTE: If the selected sgRNA does not start with G, add G at the beginning of the sgRNA to help enhance its expression. - Perform a PCR reaction to modify the pre-existing plasmid expressing sgRNA (Figure 3A) that targets Toxoplasma uracil phosphoribosyltransferase (TgUPRT) gene23 using a PCR premix with the settings provided in Table 2.
- Run the PCR product on an agarose gel to confirm successful amplification. A 10 kb PCR product is expected to be amplified (Figure 3B).
- Extract the PCR product using a DNA gel extraction kit and circularize it using a site-directed mutagenesis kit. Refer to Table 3 for the recipe. Incubate the reaction for 10-20 min at RT.
- Transform the circularized PCR product into E. coli and pick 10 clones for further verification of incorporation of designed sgRNA.
- Grow two clones and extract plasmids. Cut the purified plasmids with BamHI and EcoRV. The candidate plasmids will yield two bands at 2.4 kb and 7.2 kb (Figure 3C).
- Send the plasmids for Sanger sequencing using M13 reverse primers to confirm successful replacement of TgUPRT sgRNA with the designed sgRNA (Figure 3D).
- Go to www.ToxoDB.org and retrieve the entire gene coding sequence, including introns and exons, along with 1.5 kb 5'-UTRs and 3'-UTRs (untranslated regions).
- Generation of repair template for gene deletion via HDR mechanism
- According to the targeting sites of the selected sgRNA, locate 50 bp of 5'-UTRs or 3'-UTRs of the target gene for homology-dependent recombination (HDR, see discussion section). The selection of regions follows the criteria listed below, depending on the location the sgRNA targets.
- If the cleavage site by Cas9 is located upstream from the start codon, select the following: a 50 bp DNA sequence upstream from the cleavage site as the left HDR region, and a 50 bp DNA sequence downstream from the stop codon as the right HDR region.
- If the cleavage site by Cas9 is between the start and stop codons, select the following: a 50 bp DNA sequence upstream from the start codon as the left HDR region, and a 50 bp DNA sequence downstream from the stop codon as the right HDR region.
- If the cleavage site by Cas9 is located downstream from the stop codon, select the following: a 50 bp DNA sequence upstream from the start codon as the left HDR region, and a 50 bp DNA sequence downstream from the cleavage site as the right HDR region.
NOTE: For the TgCPL gene, the cleavage site is located between the start and stop codons. Thus, the following primers are designed for amplifying the repair template using pMDC64 as the template, which encodes a pyrimethamine resistance cassette. The sequences in black anneal to the pMDC64 plasmid for PCR amplification. The regions labeled in red are TgCPL-specific sequences for homologous recombination.
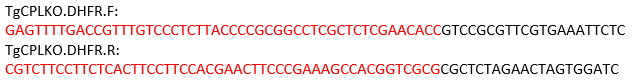
- Perform PCR using a PCR premix under the PCR conditions described in Table 4.
- Run the PCR product on an agarose gel (Figure 3E), followed by gel extraction and standard nucleic acid quantification procedures.
NOTE: If the expected band cannot be successfully amplified, optimize PCR conditions and/or switch primer pairs.
- According to the targeting sites of the selected sgRNA, locate 50 bp of 5'-UTRs or 3'-UTRs of the target gene for homology-dependent recombination (HDR, see discussion section). The selection of regions follows the criteria listed below, depending on the location the sgRNA targets.
- Toxoplasma transfection
- Pass RHΔku80::NLuc parasites for 2 days in a T25 flask containing confluent HFFs. A T25 flask of fully lysed parasites is sufficient for two to three transfections.
- Syringe and filter-purify parasites as described in step 1.2. Resuspend parasites in cytomix buffer and spin down at 1,000 x g for 10 min at RT.
- Wash pelleted parasites with 10 mL of cytomix buffer and spin down the parasites at 1,000 x g for 10 min at RT.
- Carefully pour off the supernatant and resuspend the parasites in the same buffer at a concentration of 1 x 108 parasites/mL.
- Mix 2 µg of repair template DNA with 20 µg of the sgRNA/Cas9 expression plasmids (mass ratio = 1:5, equivalent to a 1:3 molar ratio). If the amplification yield of repair template is low, reduce the input of both DNA pieces accordingly. A minimum of 0.5 µg of repair template can be used.
- Mix 400 µL of parasite resuspension, DNA, and 5 µL of 200 mM ATP/500 mM reduced glutathione (GSH) in a 1.5 mL centrifuge tube. Bring the total volume to 500 µL with cytomix buffer, if needed.
- Transfer the mixture of parasites and DNA to an electroporation cuvette (4 mm gap width) and perform electroporation (2 kV voltage, 50 Ω resistance) using an electroporation apparatus.
- Transfer electroporated parasites to a T25 flask containing confluent HFFs in fresh D10 medium. Apply appropriate antibiotic for drug selection after 24 h.
- Keep drug selective pressure until the growth of the transgenic parasites is stable.
- Purify genomic DNA from the knockout population and check for integration of the pyrimethamine resistance cassette into the TgCPL locus by PCR. After verified, proceed to section 3.4. If not, perform another round of parasite transfection and drug selection. Inability to detect the correct integration of the drug resistance cassette usually suggests that the target gene is essential or that the gene locus is not accessible.
- Cloning of knockout parasites
- Seed two 96 well microplates with HFF cells and incubate at 37 °C and 5% CO2 for 1 week prior to cloning parasites.
- Pass ~0.3-0.4 mL of the population of transgenic parasites in a T25 flask containing confluent HFFs and grow them for 2 days. Consider passing more parasites if the mutant shows growth defects.
NOTE: To achieve the best yield and viability, the host cells are heavily infected by the parasites, and most of the parasites are kept in the intracellular stage. - Syringe infected host cells and filter-purify freshly lysed parasites as mentioned in step 1.3. Resuspend the parasites in D10 medium and spin them down at 1,000 x g for 10 min at RT.
- Resuspend the pelleted parasites in 10 mL of D10 medium.
- Count parasites using a hemocytometer to determine the parasite concentration.
- Conduct a two-step dilution to bring the concentration to 10 parasites/mL in D10 medium supplemented with the appropriate antibiotic. Usually, the initial parasite resuspension is diluted by 1,000-fold, followed by a second dilution to 10 parasites/mL.
- Aspirate media from 96 well microplates containing confluent HFFs and inoculate 150 µL of diluted parasites into each well.
- Incubate plates at 37 °C with 5% CO2 for 7 days without disturbance to allow plaque formation. The incubation period can be longer if transgenic parasites exhibit growth defects.
- Screen the plates using a phase-contrast microscope and mark only the wells containing a single plaque.
- Perform colony PCR to identify correct clones.
- Use pipette tips to scrape the bottom of each well to lift infected HFF monolayers.
- Pipet 75 µL of the cell resuspension from each marked well into 1.5 mL microcentrifuge tubes.
- Centrifuge tubes for 10 min at maximum speed at RT. Carefully aspirate the supernatant and resuspend the pellet in 10.25 µL of lysis buffer containing dilution buffer and DNA release additive provided in the kit (Table of Materials).
- Incubate the samples for 4 min at RT, then 2 min at 98 °C. Afterward, samples can be used for PCR or stored at -20 °C until use. Three sets of PCR reactions are used to test for the integration of the drug resistance cassette and loss of the gene of interest (Figure 4A). Refer to Table 5 for PCR reaction setup and Table 6 for thermocycler settings.
- Identify the correct clones and transfer four clones into T25 flasks containing confluent HFFs.
- After individual clones lyse host cells, purify genomic DNA for further PCR verification.
- If an antibody recognizing the protein of interest is available, follow a standard immunoblotting procedure to verify loss of the target protein in the correct Toxoplasma knockouts. Representative images for screening a TgCPL-deletion mutant are shown in Figure 4B,C.
Wyniki
Figure 1 represents an example of a growth curve for the RHΔku80::NLuc strain and the derived calculation for its doubling time. Generally, the assay is performed in three technical replicates for each of the three biological replicates to account for variations of luciferase activity readings. In order to calculate the normalized fold change of parasite growth, each reading at 24-96 h post-infection was divided by the initial reading a...
Dyskusje
++This protocol describes a luciferase-based protocol to assess intracellular Toxoplasma growth and evaluate the inhibition efficacy of chemical compounds against parasite growth. Compared to the existing strategies available for measuring intracellular Toxoplasma growth, this method exhibits high sensitivity and specificity. While monitoring parasite growth, a mock assay in a clear 96 well microplate is recommended to confirm that the tested strain does not prematurely lyse host cells before the end of...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors would like to thank Drs. Sibley and Carruthers for sharing pSAG1-Cas9-sgRNA-TgUPRT plasmid and anti-TgCPL and TgActin antibodies. This work was supported by the Clemson Startup fund (to Z.D.), Knights Templar Eye Foundation Pediatric Ophthalmology Career-Starter Research Grant (to Z.D.), a pilot grant of an NIH COBRE grant P20GM109094 (to Z.D.), and NIH R01AI143707 (to Z.D.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materiały
| Name | Company | Catalog Number | Comments |
| Agarose gel extraction kit | New England BioLabs | T1020L | |
| BamHI | New England BioLabs | R0316S | |
| Biotek Synergy H1 Hybrid Multi-Mode Microplate Reader | BioTek Instuments | ||
| BTX Gemini Twin Waveform Electroporation System | Harvard Apparatus | ||
| Chemically competent E. coli cells | New England BioLabs | C29871 | |
| CloneAmp HiFi PCR premix | Takara Bio | 639298 | |
| Coelenterazine h | Prolume | 301-10 hCTZ | |
| EcoRV | New England BioLabs | R3195S | |
| Phire Tissue Direct PCR Master Mix | Thermo Scientific | F170L | |
| Plasmid miniprep kit | Zymo Research | D4054 | |
| Q5 Site-Directed Mutagenesis kit | New England BioLabs | E0554S | |
| Software | |||
| Geneious software for sgRNA design (version: R11) | |||
| GraphPad Prism software (8th version) | |||
| SnapGene for molecular cloning (version: 4.2.11) |
Odniesienia
- Blader, I. J., Coleman, B. I., Chen, C. T., Gubbels, M. J. Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annual Review of Microbiology. 69 (1), 1-23 (2014).
- Jones, J. L., Kruszon-Moran, D., Rivera, H., Price, C., Wilkins, P. P. Toxoplasma gondii Seroprevalence in the United States 2009-2010 and Comparison with the Past Two Decades. The American Journal of Tropical Medicine and Hygiene. 90 (6), (2014).
- Kieffer, F., Wallon, M. Congenital toxoplasmosis. Handbook of Clinical Neurology. 112, 1099-1101 (2013).
- Hoffmann, S., Batz, M. B., Morris, G. J. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. Journal of Food Protection. 75 (7), 1292-1302 (2012).
- Dubey, J. Toxoplasmosis. Journal of the American Veterinary Medical Association. 205 (11), 1593-1598 (1994).
- Lindsay, D., Dubey, J. Toxoplasma gondii: the changing paradigm of congenital toxoplasmosis. Parasitology. 138 (14), 1-3 (2011).
- Deng, Y., Wu, T., Zhai, S., Li, C. Recent progress on anti-Toxoplasma drugs discovery: Design, synthesis and screening. European Journal of Medicinal Chemistry. 183, 111711 (2019).
- Butler, N. J., Furtado, J. M., Winthrop, K. L., Smith, J. R. Ocular toxoplasmosis II: clinical features, pathology and management. Clinical & Experimental Ophthalmology. 41 (1), 95-108 (2013).
- Pfefferko, E., Pfefferko, L. C. Specific Labeling of Intracellular Toxoplasma gondii with Uracil. Journal of Eukaryotic Microbiology. 24 (3), 449-453 (1977).
- Merli, A., Canessa, A., Melioli, G. Enzyme immunoassay for evaluation of Toxoplasma gondii growth in tissue culture. Journal of Clinical Microbiology. 21 (1), 88-91 (1985).
- Derouin, F., Chastang, C. Enzyme immunoassay to assess effect of antimicrobial agents on Toxoplasma gondii in tissue culture. Antimicrobial Agents and Chemotherapy. 32 (3), 303-307 (1988).
- McFadden, D., Seeber, F., Boothroyd, J. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrobial Agents and Chemotherapy. 41 (9), 1849-1853 (1997).
- Gubbels, M. J., Li, C., Striepen, B. High-Throughput Growth Assay for Toxoplasma gondii Using Yellow Fluorescent Protein. Antimicrobial Agents and Chemotherapy. 47 (1), 309-316 (2003).
- Touquet, B., et al. High-content imaging assay to evaluate Toxoplasma gondii infection and proliferation: A multiparametric assay to screen new compounds. PLoS ONE. 13 (8), e0201678 (2018).
- Thornton, L. B., et al. An ortholog of Plasmodium falciparum chloroquine resistance transporter (PfCRT) plays a key role in maintaining the integrity of the endolysosomal system in Toxoplasma gondii to facilitate host invasion. PLOS Pathogens. 15 (6), e1007775 (2019).
- Larson, E. T., et al. Toxoplasma gondii cathepsin L is the primary target of the invasion-inhibitory compound morpholinurea-leucyl-homophenyl-vinyl sulfone phenyl. The Journal of Biological Chemistry. 284 (39), 26839-26850 (2009).
- Dou, Z., McGovern, O. L., Cristina, M., Carruthers, V. B. Toxoplasma gondii Ingests and Digests Host Cytosolic Proteins. mBio. 5 (4), e01188-14 (2014).
- Cristina, M., et al. Toxoplasma depends on lysosomal consumption of autophagosomes for persistent infection. Nature Microbiology. 2, 17096 (2017).
- Parussini, F., Coppens, I., Shah, P. P., Diamond, S. L., Carruthers, V. B. Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii. Molecular Microbiology. 76 (6), 1340-1357 (2010).
- Huynh, M. H., Carruthers, V. B. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryotic cell. 8 (4), 530-539 (2009).
- Fox, B. A., Ristuccia, J. G., Gigley, J. P., Bzik, D. J. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryotic Cell. 8 (4), 520-529 (2009).
- Sidik, S. M., Hackett, C. G., Tran, F., Westwood, N. J., Lourido, S. Efficient Genome Engineering of Toxoplasma gondii Using CRISPR/Cas9. PLoS ONE. 9 (6), e100450 (2014).
- Shen, B., Brown, K. M., Lee, T. D., Sibley, D. L. Efficient Gene Disruption in Diverse Strains of Toxoplasma gondii Using CRISPR/CAS9. mBio. 5 (3), e01114-14 (2014).
- Radke, J. R., et al. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Molecular and Biochemical Parasitology. 115 (2), 165-175 (2001).
- Ran, A. F., et al. Genome engineering using the CRISPR-Cas9 system. Nature Protocols. 8 (11), 2281-2308 (2013).
- Labun, K., Montague, T. G., Gagnon, J. A., Thyme, S. B., Valen, E. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Research. 44 (W1), W272-W276 (2016).
- Heigwer, F., Kerr, G., Boutros, M. E-CRISP: fast CRISPR target site identification. Nature Methods. 11 (2), 2812 (2014).
- Peng, D., Tarleton, R. EuPaGDT: a web tool tailored to design CRISPR guide RNAs for eukaryotic pathogens. Microbial Genomics. 1 (4), e000033 (2015).
- Doench, J. G., et al. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nature Biotechnology. 32 (12), 1262-1267 (2014).
- Sidik, S. M., et al. A Genome-wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell. 166 (6), 1423-1435 (2016).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone