Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Characterization of Intra-Cartilage Transport Properties of Cationic Peptide Carriers
W tym Artykule
Podsumowanie
This protocol determines equilibrium uptake, depth of penetration and non-equilibrium diffusion rate for cationic peptide carriers in cartilage. Characterization of transport properties is critical for ensuring an effective biological response. These methods can be applied for designing an optimally charged drug carriers for targeting negatively charged tissues.
Streszczenie
Several negatively charged tissues in the body, like cartilage, present a barrier to the targeted drug delivery due to their high density of negatively charged aggrecans and, therefore, require improved targeting methods to increase their therapeutic response. Because cartilage has a high negative fixed charge density, drugs can be modified with positively charged drug carriers to take advantage of electrostatic interactions, allowing for enhanced intra-cartilage drug transport. Studying the transport of drug carriers is, therefore, crucial towards predicting the efficacy of drugs in inducing a biological response. We show the design of three experiments which can quantify the equilibrium uptake, depth of penetration and non-equilibrium diffusion rate of cationic peptide carriers in cartilage explants. Equilibrium uptake experiments provide a measure of the solute concentration within the cartilage compared to its surrounding bath, which is useful for predicting the potential of a drug carrier in enhancing therapeutic concentration of drugs in cartilage. Depth of penetration studies using confocal microscopy allow for the visual representation of 1D solute diffusion from the superficial to deep zone of cartilage, which is important for assessing whether solutes reach their matrix and cellular target sites. Non-equilibrium diffusion rate studies using a custom-designed transport chamber enables the measurement of the strength of binding interactions with the tissue matrix by characterizing the diffusion rates of fluorescently labeled solutes across the tissue; this is beneficial for designing carriers of optimal binding strength with cartilage. Together, the results obtained from the three transport experiments provide a guideline for designing optimally charged drug carriers which take advantage of weak and reversible charge interactions for drug delivery applications. These experimental methods can also be applied to evaluate the transport of drugs and drug-drug carrier conjugates. Further, these methods can be adapted for the use in targeting other negatively charged tissues such as meniscus, cornea and the vitreous humor.
Wprowadzenie
Drug-delivery to negatively charged tissues in the body remains a challenge due to the inability of drugs to penetrate deep into the tissue to reach cell and matrix target sites1. Several of these tissues comprise of densely-packed, negatively-charged aggrecans which create a high negative fixed charge density (FCD)2 within the tissue and act as a barrier for the delivery of most macromolecules3,4. However, with the assistance of positively charged drug carriers, this negatively charged tissue barrier can actually be converted into a drug depot via electrostatic charge interactions for sustained drug delivery1,5,6,7(Figure 1).
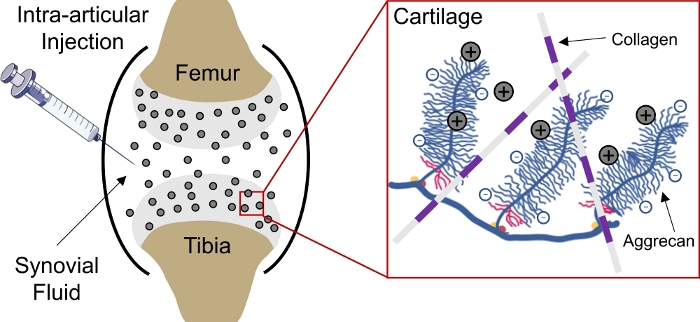
Figure 1: Charge based intra-cartilage delivery of CPCs. Intra-articular injection of CPCs into the knee joint space. Electrostatic interactions between positively charged CPCs and negatively charged aggrecan groups enable rapid and full depth penetration through cartilage. This figure has been modified from Vedadghavami et al4. Please click here to view a larger version of this figure.
Recently, short-length cationic peptide carriers (CPCs) were designed with the goal of creating small cationic domains capable of carrying larger sized therapeutics for delivery to the negatively charged cartilage4. For effective drug delivery to the cartilage for treating prevalent8,9 and degenerative diseases such as osteoarthritis (OA)10, it is critical that therapeutic concentrations of drugs penetrate deep within the tissue, where a majority of the cartilage cells (chondrocytes) lie11. Although there are several potential disease modifying drugs available, none have gained FDA approval because these are unable to effectively target the cartilage12,13. Therefore, evaluation of the transport properties of drug carriers is necessary for predicting the effectiveness of drugs in inducing a therapeutic response. Here, we have designed three separate experiments that can be utilized for assessing the equilibrium uptake, depth of penetration and non-equilibrium diffusion rate of CPCs4.
To ensure that there is a sufficient drug concentration within the cartilage that can provide an optimal therapeutic response, uptake experiments were designed to quantify equilibrium CPC concentration in cartilage4. In this design, following an equilibrium between the cartilage and its surrounding bath, the total amount of solute inside the cartilage (either bound to the matrix or free) can be determined using an uptake ratio. This ratio is calculated by normalizing the concentration of solutes inside the cartilage to that of the equilibrium bath. In principle, neutral solutes, whose diffusion through the cartilage is not assisted by charge interactions, would have an uptake ratio of less than 1. Conversely, cationic solutes, whose transport is enhanced via electrostatic interactions, show an uptake ratio greater than 1. However, as shown with CPCs, use of an optimal positive charge can result in much higher uptake ratios (greater than 300)4.
Although high drug concentration within the cartilage is important for achieving therapeutic benefit, it is also critical that drugs diffuse through the full thickness of the cartilage. Therefore, studies showing the depth of penetration are required to ensure that drugs reach deep within the cartilage so that the matrix and cellular target sites can be reached, thereby providing a more effective therapy. This experiment was designed to assess the one-way diffusion of solutes through cartilage, simulating diffusion of drugs into cartilage following intra-articular injection in vivo. Fluorescence imaging using confocal microscopy allows for the evaluation of depth of penetration into cartilage. Net particle charge plays a key role in moderating how deep drugs can diffuse through the matrix. An optimal net charge based on a tissue FCD is required to allow for weak-reversible binding interactions between cationic particles and the anionic tissue matrix. This implies that any interaction is weak enough so that particles can disassociate from the matrix but reversible in nature so that it can bind to another matrix binding site deeper within the tissue4. Conversely, excessive positive net charge of a particle can be detrimental towards diffusion, as too strong matrix binding prevents detachment of particles from the initial binding site in the superficial zone of cartilage. This would result in an insufficient biological response as a majority of the target sites lie deep within the tissue11.
To further quantify the strength of the binding interactions, analysis of drug diffusion rates through cartilage is advantageous. Non-equilibrium diffusion studies allow for the comparison of real-time diffusion rates between different solutes. As drugs diffuse through the superficial, middle and deep zones of cartilage, the presence of binding interactions can greatly alter diffusion rates. When binding interactions are present between drugs and the cartilage matrix, it is defined as the effective diffusivity (DEFF). In this case, once all binding sites have been occupied, the diffusion rate of drugs is governed by the steady-state diffusion (DSS). Comparison between the DEFF of different solute determines the relative binding strength of solutes with the matrix. For a given solute, if the DEFF and DSS are within the same order of magnitude, it implies that there is minimal binding present between the drug and matrix during diffusion. However, if DEFF is greater than DSS, substantial binding of particles to matrix exists.
The designed experiments individually allow for the characterization of solute transport through the cartilage, however, a holistic analysis inclusive of all results is required for designing an optimally charged drug carrier. The weak and reversible nature of charge interactions controls particle diffusion rate and allows for high equilibrium uptake and rapid full depth penetration through cartilage. Through equilibrium uptake experiments, we should look for carriers that show high uptake as a result of charge interactions which can be verified using non-equilibrium diffusion rate studies. However, these binding interactions should be weak and reversible in nature to allow for full-thickness penetration of the solute through cartilage. An ideal drug carrier would possess an optimal charge which enables strong enough binding for uptake and high intra-cartilage drug concentrations, but not too strong as to impede full-thickness diffusion4. The presented experiments will assist in the design characteristics for charge-based tissue targeting drug carriers. These protocols were used for characterizing CPC transport through cartilage4, however, these can also be applied to a variety of drugs and drug carriers through cartilage and other negatively charged tissues.
Protokół
University approvals were obtained for conducting the experiments with dead tissues. Bovine joints were obtained commercially from a slaughterhouse.
1. Cartilage explant extraction
- Using a scalpel (#10 blade), cut and remove fat, muscles, ligaments, tendons and all other connective tissue to expose the cartilage from the femoropatellar groove of bovine knee joints.
- Using 3 mm and 6 mm dermal punches, make perpendicular punches into the cartilage to extract cylindrical plugs. Immediately place the plugs in individual wells of a 48-well plate containing 500 μL of 1x phosphate buffered saline (PBS) supplemented with 1% v/v antibiotic-antimycotic.
- Place the superficial side of a cartilage plug facing down into a well in the slicing fixture (Figure 2). Using a razor blade, slice the plug along the surface of the slicing fixture to obtain a 1 mm thick cartilage explant that is inclusive of the superficial zone. Repeat for each cartilage plug.
- Store cartilage explants individually in polypropylene tubes containing 500 μL of 1x PBS supplemented with protease inhibitors (PBS-PI, 1 PI mini-tablet per 50 mL 1x PBS) at -20 °C.
- Prior to conducting each of the following transport experiments, thaw the explant-containing vials for 30 min in a 37 °C water bath.
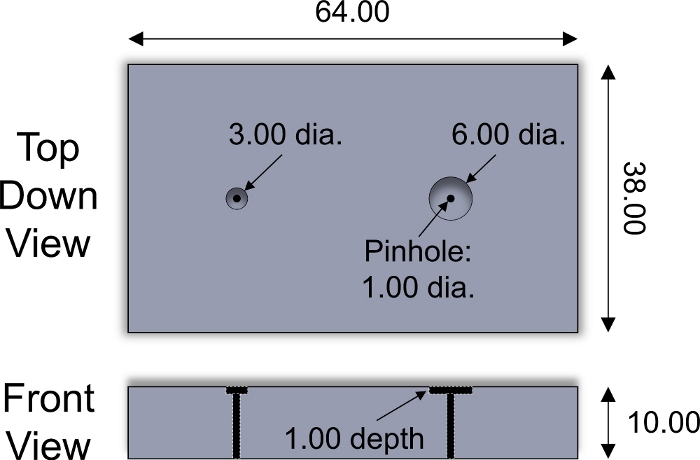
Figure 2: Custom-designed slicing fixture. Design parameters of stainless steel slicing fixture used for slicing cartilage explants of 3 and 6 mm diameter. Plastic inserts of varying thickness were placed inside wells to adjust the thickness of sliced explants. Stainless steel cylindrical pin of <1 mm diameter was used to push explant out of fixture. All numerical values are presented in mm. Please click here to view a larger version of this figure.
2. Equilibrium uptake of CPCs in cartilage
- Gently dab cartilage explants (3 mm dia. X 1 mm thick.) with a delicate task wipe to remove excess 1x PBS from the explant surface. Using a balance, quickly record the wet weight of each explant and then immediately place in a 1x PBS bath to prevent dehydration.
- Prepare 30 μM solutions (300 μL per explant) of fluorescently labeled CPCs in 1x PBS-PI. Use RNase-free polypropylene tubes for reconstitution.
- In a 96-well plate, pipette 300 μL of each 30 μM CPC solution into separate wells. Avoid using wells near the edge of the plate to prevent evaporation. Using a spatula, transfer each explant to the solution containing wells.
- Fill surrounding wells with 300 μL of 1x PBS and cover the well plate with lid. Seal the edges of the plate with flexible film to minimize evaporation.
- Inside of a 37 °C incubator, place the plate on a plate shaker to limit the particle sedimentation. Incubate for 24 h under gentle rotation (50 rpm with a 15 mm orbit) to allow for the equilibrium uptake of CPCs in the cartilage (Figure 3).
- Generate a standard curve for correlation of fluorescence to CPC concentration
- Prepare serial dilutions of CPC solutions from 30 μM – 0 μM (10 2-fold dilutions) in 1x PBS-PI in polypropylene tubes. Ensure that at least 500 μL of each dilution is present.
- Add 200 μL of each dilution to consecutive wells in a black 96-well plate. Duplicate in another row to increase sample size.
- Obtain fluorescence readings of each sample using a plate reader at the excitation and emission wavelengths of the fluorescent label using a plate reader.
- Plot fluorescence reading vs. CPC concentration and derive an equation for the linear portion of the curve.
NOTE: To limit the variability in fluorescence readings, incubate the CPC stock solution under the same conditions as the sample plate prior to generation of the standard curve.
- After 24 h of incubation, collect the equilibrium bath from each well in separate polypropylene tubes.
- Transfer 200 μL of each solution into separate wells of a black 96-well plate. Obtain fluorescence readings of each sample under the same fluorescent settings as for the standard curve. If necessary, dilute the sample in 1x PBS-PI to ensure readings fall within the linear portion of the standard curve.
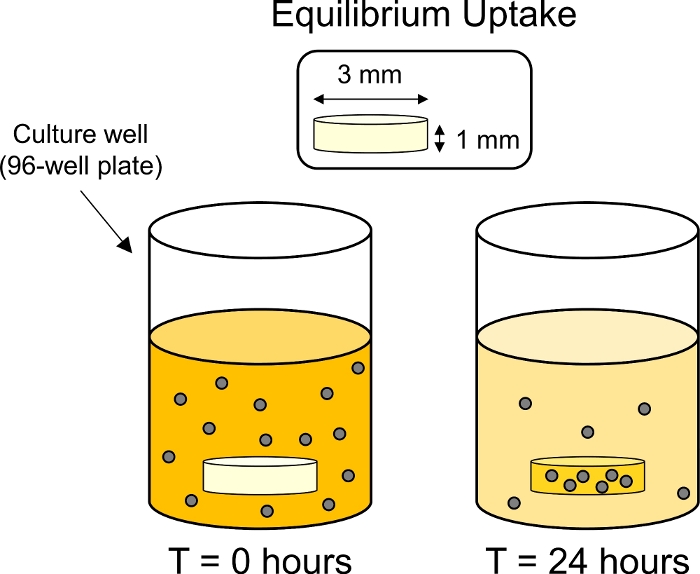
Figure 3: Schematic of equilibrium uptake experiments. Cartilage explants (3 mm dia. x 1 mm thick) were placed in individuals wells in a 96-well plate containing fluorescently tagged CPC solution. After 24 h CPCs were uptaken by the cartilage, thereby reducing the fluorescence of the surrounding bath. Please click here to view a larger version of this figure.
3. Depth of penetration of CPCs in the cartilage
- Prepare 30 μM solutions (300 μL per explant) of fluorescently labeled CPCs in 1x PBS-PI. Use RNase-free polypropylene tubes for reconstitution.
- Using a scalpel, cut cartilage explants (6 mm diameter x 1 mm thickness) in half to make half-disks. Keep the explant hydrated with a layer of 1x PBS-PI while cutting.
- Glue a half-disk explant into the middle of one well of the custom-designed 1-dimensional transport chamber using an epoxy (Figure 4, Figure 5). Ensure epoxy is applied to the circumferential (curved) side of the explant. Remove excess glue from the well to prevent contact with the diffusion surface area of cartilage and make a note of the superficial side of the explant.
- Add 80 μL of 1x PBS-PI to both sides of the explant. Pipette the liquid up and down from one side of the explant to check for leakage to the other side. If leakage occurs, readjust explant and apply epoxy as needed.
- Replace the 1x PBS-PI from the side facing the superficial surface of cartilage (upstream) with 80 μL of 30 μM CPC solution. Maintain 80 μL of 1x PBS-PI on the side facing the deep zone of cartilage (downstream).
- Carefully place the transport chamber in a coverable container. Cover the base of the container with a layer 1x PBS to avoid evaporation of solutions. Ensure that there is no direct contact between solutions from upstream and downstream chambers.
- Place the covered container on a plate shaker to limit particle sedimentation. Incubate for either 4 or 24 h at room temperature under gentle rotation (50 rpm with a 15 mm orbit).
- After incubation, remove the explant from chamber and cut ~100 μm slice from the center of the explant.
NOTE: This cross-section is inclusive of the superficial, middle and deep zones of cartilage. - Place the slice between a glass slide and a coverslip. Hydrate the slice with a layer of 1x PBS-PI.
- At 10x magnification, image through the full thickness of the slice to obtain z-stack of fluorescent images using a confocal microscope.
- Using ImageJ project the average intensity of the images within the z-stack to determine the depth of penetration of CPCs in cartilage.
- Open the image stack by clicking on File | Open.
- Click on ‘Image’ on the task bar and click Image | Stacks | Z Project from the dropdown menu.
- Input slice numbers from 1 to the final slice. Select ‘Average Intensity’ under Projection Type. Click ‘OK.’
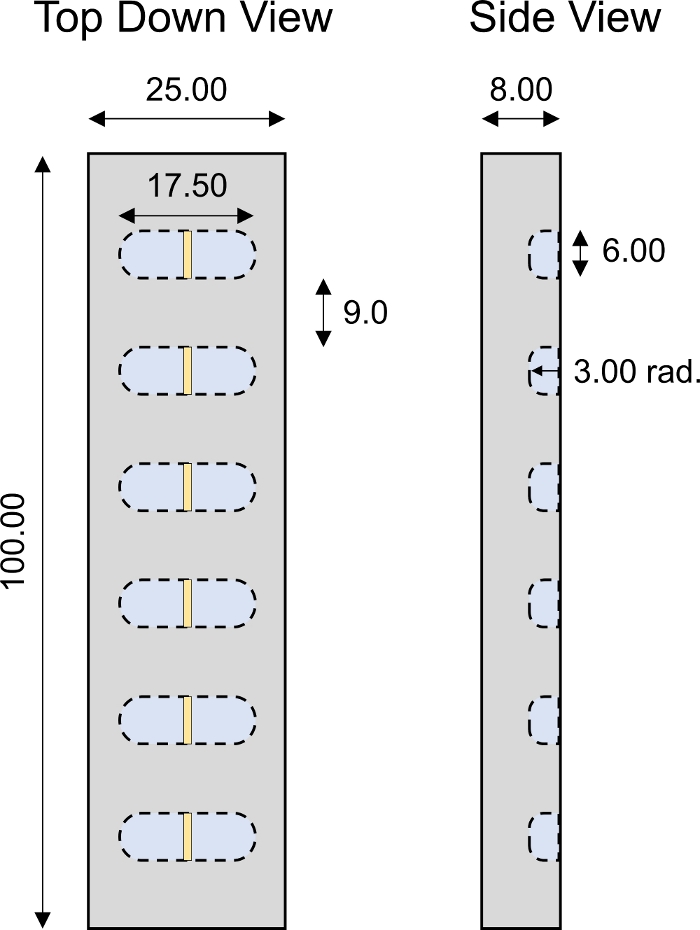
Figure 4: Custom-designed 1-D transport chamber. Design parameters of PMMA 1D transport chamber with 6 individual wells. All numerical values are presented in mm. Please click here to view a larger version of this figure.
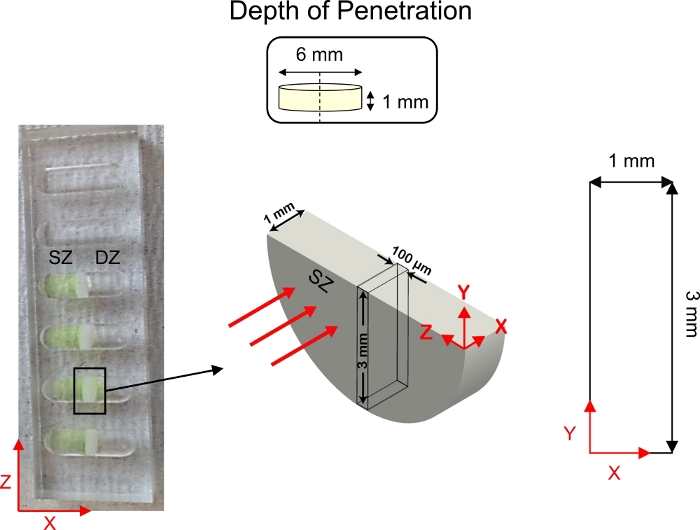
Figure 5: Schematic of depth of penetration studies. Cartilage explants (6 mm diameter x 1 mm thickness) were cut in half and fixed to the center of 1-D diffusive transport wells. Fluorescently tagged CPC solution was added to the side of the well in contact with the superficial zone (SZ) of cartilage. 1x PBS-PI was added to the side of the well in contact with the deep zone (DZ) of cartilage. Following diffusion, a cross-section of cartilage (3 mm x 1 mm) was imaged using confocal microscopy. This figure has been modified from Vedadghavami et al.4 and Bajpayee et al.3 Please click here to view a larger version of this figure.
4. Non-equilibrium diffusion rate of CPCs in the cartilage
- Bring the two halves of the custom-designed transport chamber (Figure 6) together to assemble and close the chamber. Use washers, nuts and bolts to tightly close the chamber with a wrench.
NOTE: The transport chamber must be translucent as to not interfere with fluorescent readings. The transport chambers used in this protocol are made from polymethylmethacrylate (PMMA). - Coat the inner space of the chamber with 0.5% w/v non-fat bovine milk solution in 1x PBS (2 mL for each chamber) for 15 min to prevent non-specific binding of CPCs to chamber walls. Then rinse the chamber with 1x PBS (2 mL for each chamber).
- Using the custom-designed slicing fixture (Figure 2) and a razor blade, slice a 6 mm diameter cartilage explant (transverse plane) to a thickness of 500-800 μm, inclusive of the superficial zone. Keep the explant hydrated with 1x PBS.
- Using hammer-driven and dermal punches, create gaskets from rubber sheets as shown in Figure 7.
- Assemble each half transport chamber to include 1 large rubber gasket, 1 PMMA insert and 1 small rubber gasket each. Place the explant in the wells of the plastic insert, with the superficial zone facing the upstream chamber. Sandwich the two halves together to complete the assembly and screw tightly using a wrench (Figure 7).
- Fill the upstream chamber with 2 mL of 1x PBS-PI and observe the downstream chamber for leakage of fluid from the upstream chamber. If leakage is present, reassemble the chamber, adjusting gasket position and tightness of screws. If no leakage, fill the downstream chamber with 2 mL 1x PBS-PI as well.
- Add a mini-stir bar to both up and downstream chambers and place the chamber on a stir plate. Align the chamber so that laser from the spectrophotometer is focused towards the center of the downstream chamber. Place the signal receiver portion of the spectrophotometer behind the downstream chamber (Figure 8).
NOTE: The laser and receiver of the spectrophotometer must be equipped with the appropriate filters to excite, emit and transmit signals from the fluorescently labeled protein. Protect the transport chamber from light using a black box during experimentation to avoid interference in fluorescence signal. It is best practice to seal the openings on top of the chamber with flexible film to avoid evaporation. - Collect real-time downstream fluorescence emission readings and ensure a stable signal for at least 5 min.
NOTE: Aliquots from the downstream chamber can be obtained and assessed for fluorescence using a plate reader if a custom-designed spectrophotometer or translucent transport chamber is not available. - Pipette a pre-calculated volume of stock solution of fluorescently tagged CPCs into the upstream chamber to ensure a final concentration of 3 μM inside the upstream chamber. Observe the downstream fluorescence signal and allow for solute transport to reach a steady increase in slope.
NOTE: A thicker cartilage explant will require longer time to reach steady-state. - Once the steady state has been reached, take 20 μL from the upstream chamber and add to the downstream chamber (“spike test”).
NOTE: A spike in the downstream fluorescence will be observed. This will allow for correlation between fluorescence readings and CPC concentration. - Collect real-time downstream fluorescence readings.
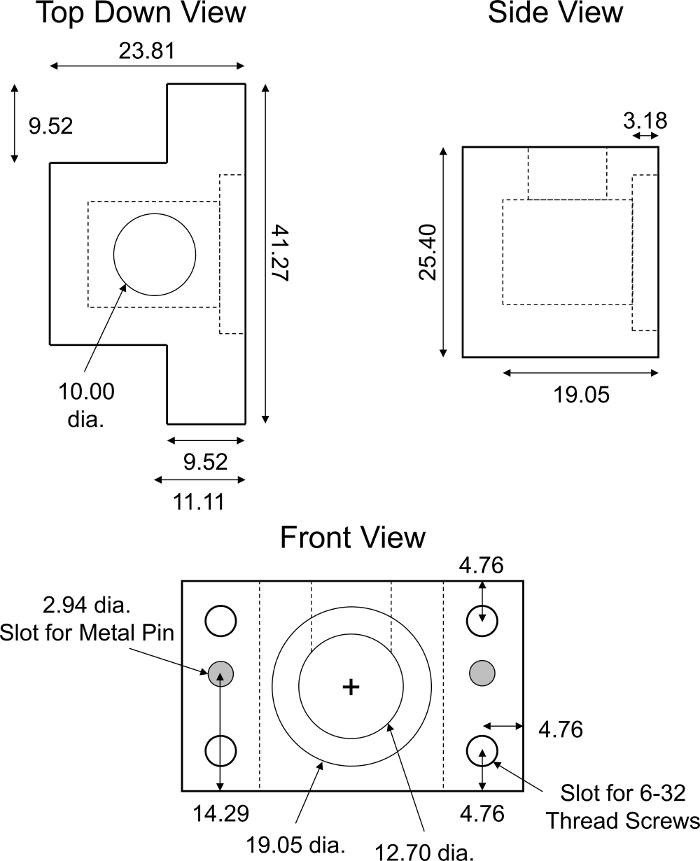
Figure 6: Custom-designed non-equilibrium diffusion transport chamber. Design parameters of PMMA non-equilibrium diffusion transport chamber. The chamber must be translucent as to not interfere with fluorescence readings. The complete transport chamber consisted of two identical halves of the fixture shown. Two cylindrical stainless-steel pins (~2.94 mm diameter, ~18 mm long) were required to ensure alignment and complete closure of the halves of the chamber. Four identical slots for 6-32 thread screws were made in each corner of the chamber for screw tight assembly. All numerical values are presented in millimeters. Please click here to view a larger version of this figure.
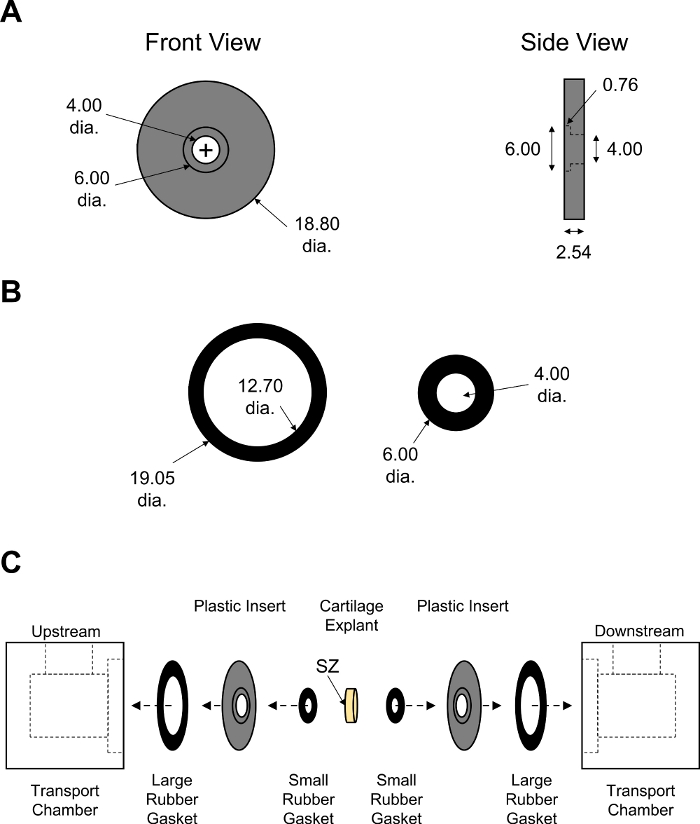
Figure 7: Assembly of non-equilibrium diffusion transport chamber. Design parameters of (A) black PMMA inserts and (B) large and small rubber gaskets. Thickness of rubber gaskets was adjusted to ensure tight closure of the chamber. All numerical values are presented in mm. (C) Schematic showing the order of assembly for two halves of transport chamber with cartilage explant placed in the center. SZ indicates superficial zone of cartilage which was facing the upstream chamber. Please click here to view a larger version of this figure.
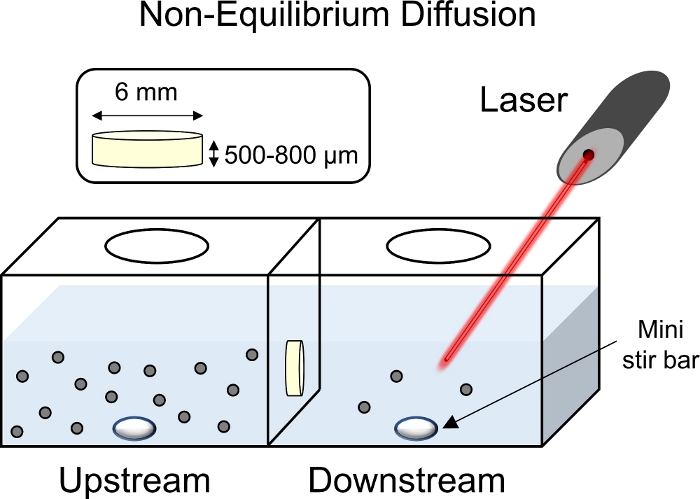
Figure 8: Schematic of non-equilibrium diffusion experiments. Cartilage explants (6 mm diameter x 1 mm thickness) were placed in the center of the transport chamber with the superficial surface facing the upstream chamber. Both up and downstream sides of the chamber were filled with 1x PBS-PI and mixed using a mini stir bar. With a laser pointed towards the downstream chamber to collect fluorescent readings, fluorescently tagged CPC solution was added to the upstream chamber. Please click here to view a larger version of this figure.
Wyniki
Following equilibrium absorption of CPCs by cartilage, the bath fluorescence decreases when the solute has been uptaken by the tissue. However, if the fluorescence value of the final bath remains similar to the initial, it indicates that there is no/minimal solute uptake. Another confirmation of solute uptake is if the tissue has visibly changed color to the color of the fluorescent dye. The quantitative uptake of solutes in cartilage was determined using the uptake ratio (RU) after the fluorescence values wer...
Dyskusje
The methods and protocols described here are significant to the field of targeted drug delivery to negatively charged tissues. Due to the high density of negatively charged aggrecans present in these tissues, a barrier is created, thus preventing drugs from reaching their cellular target sites which lie deep within the matrix. To address this outstanding challenge, drugs can be modified to incorporate positively charged drug carriers which can enhance the transport rate, uptake and binding of drugs within tissue
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was funded by the United States Department of Defense through the Congressionally Directed Medical Research Programs (CDMRP) under contract W81XWH-17-1-0085, and the National Institute of Health R03 EB025903-1. AV was funded by the College of Engineering Dean’s Fellowship at Northeastern University.
Materiały
| Name | Company | Catalog Number | Comments |
| 316 Stainless Steel SAE Washer | McMaster-Carr | 91950A044 | For number 5 screw size, 0.14" ID, 0.312" OD |
| 96-Well Polystyrene Plate | Fisherbrand | 12566620 | Black |
| Acrylic Thick Gauge Sheet | Reynolds Polymer | N/A | For non-equilibrium diffusion and 1-D diffusion transport chamber |
| Antibiotic-Antimycotic | Gibco | 15240062 | 100x |
| Bovine Cartilage | Research 87 | N/A | 2-3 weeks old, femoropatellar groove |
| Bovine Serum Albumin | Fisher BioReagents | BP671-1 | |
| CPC+14 | LifeTein | LT1524 | Custom designed peptide |
| CPC+20 | LifeTein | LT1525 | Custom designed peptide |
| CPC+8 | LifeTein | LT1523 | Custom designed peptide |
| Delicate Task Wipers | Kimberly-Clark Professional | 34155 | |
| Dermal Punch | MedBlades | MB5-1 | 3, 4 and 6 mm |
| Economy Plain Glass Microscope Slides | Fisherbrand | 12550A3 | |
| Flat Bottom Cell Culture Plates | Corning Costar | 3595 | Clear, 96 well |
| Flexible Wrapping Film | Bemis Parafilm M Laboratory | 1337412 | |
| Gold Seal Cover Glass | Electron Microscopy Sciences | 6378701 | # 1.5, 18x18 mm |
| Hammer-Driven Hole Punch | McMaster-Carr | 3427A15 | 1/2" Diameter |
| Hammer-Driven Hole Punch | McMaster-Carr | 3427A19 | 3/4" Diameter |
| Laser | Chroma Technology | AT480/30m | Spectrophotometer Laser Light |
| Low-Strength Steel Hex Nut | McMaster-Carr | 90480A007 | 6-32 Thread size |
| LSM 700 Confocal Microscope | Zeiss | LSM 700 | |
| Micro Magnetic Stirring Bars | Bel-Art Spinbar | F37119-0007 | 7x2 mm |
| Multipurpose Neoprene Rubber Sheet | McMaster-Carr | 1370N12 | 1/32" Thickness |
| Non-Fat Dried Bovine Milk | Sigma Aldrich | M7409 | |
| Petri Dish | Chemglass Life Sciences | CGN1802145 | 150 mm diameter |
| Phosphate-Buffered Saline | Corning | 21-040-CMR | 1x |
| Plate Shaker | VWR | 89032-088 | |
| Protease Inhibitors | Thermo Scientific | A32953 | |
| Razor Blades | Fisherbrand | 12640 | |
| R-Cast Acrylic Thin Gauge Sheet | Reynolds Polymer | N/A | Black transport chamber inserts |
| RTV Silicone | Loctite | 234323 | Epoxy, Non-corrosive, clear |
| Scalpel | TedPella | 549-3 | #10, #11 blades |
| Signal Receiver | Chroma Technology | ET515lp | Spectrophotometer Laser Signal Receiver |
| Snap-Cap Microcentrifuge Tubes | Eppendorf | 22363204 | 1.5 mL |
| Spatula | TedPella | 13508 | |
| Synergy H1 Microplate Reader | Biotek | H1M | |
| Zinc-Plated Alloy Steel Socket Head Screw | McMaster-Carr | 90128A153 | 6-32 Thread size, 1" Long |
Odniesienia
- Bajpayee, A. G., Grodzinsky, A. J. Cartilage-targeting drug delivery: can electrostatic interactions help. Nature Reviews Rheumatology. 13 (3), 183-193 (2017).
- Maroudas, A. Transport of solutes through cartilage: permeability to large molecules. Journal of Anatomy. 122, 335-347 (1976).
- Bajpayee, A. G., Wong, C. R., Bawendi, M. G., Frank, E. H., Grodzinsky, A. J. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials. 35 (1), 538-549 (2014).
- Vedadghavami, A., et al. Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues. Acta Biomaterialia. 93, 258-269 (2019).
- Mehta, S., Akhtar, S., Porter, R. M., Önnerfjord, P., Bajpayee, A. G. Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Research & Therapy. 21 (1), 238 (2019).
- Vedadghavami, A., Zhang, C., Bajpayee, A. G. Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins. Nano Today. 34, 100898 (2020).
- Young, C. C., Vedadghavami, A., Bajpayee, A. G. Bioelectricity for Drug Delivery: The Promise of Cationic Therapeutics. Bioelectricity. , (2020).
- Felson, D. T. Osteoarthritis of the knee. New England Journal of Medicine. 354 (8), 841-848 (2006).
- Wieland, H. A., Michaelis, M., Kirschbaum, B. J., Rudolphi, K. A. Osteoarthritis - An untreatable disease. Nature Reviews Drug Discovery. 4 (4), 331-344 (2005).
- Martel-Pelletier, J. Pathophysiology of osteoarthritis. Osteoarthritis and Cartilage. 7 (4), 371-373 (1999).
- Sophia Fox, A. J., Bedi, A., Rodeo, S. A. The basic science of articular cartilage: Structure, composition, and function. Sports Health. 1 (6), 461-468 (2009).
- Chevalier, X., et al. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care and Research. 61 (3), 344-352 (2009).
- Cohen, S. B., et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Research and Therapy. 13 (4), 125 (2011).
- Evans, C. H., Kraus, V. B., Setton, L. A. Progress in intra-articular therapy. Nature Reviews Rheumatology. 10 (1), 11-22 (2014).
- He, T., et al. Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. Journal of Controlled Release. 318, 109-123 (2020).
- Bajpayee, A. G., Scheu, M., Grodzinsky, A. J., Porter, R. M. A rabbit model demonstrates the influence of cartilage thickness on intra-articular drug delivery and retention within cartilage. Journal of Orthopaedic Research. 33 (5), 660-667 (2015).
- Bajpayee, A. G., Quadir, M. A., Hammond, P. T., Grodzinsky, A. J. Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthritis and Cartilage. 24 (1), 71-81 (2016).
- Zhang, C., et al. Avidin-biotin technology to synthesize multi-arm nano-construct for drug delivery. MethodsX. , 100882 (2020).
- Wagner, E. K., et al. Avidin grafted dextran nanostructure enables a month-long intra-discal retention. Scientific Reports. 10.1, 1-14 (2020).
- Troeberg, L., Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochimica et Biophysica Acta - Proteins and Proteomics. 1824 (1), 133-145 (2012).
- Kirk, T. B., Wilson, A. S., Stachowiak, G. The effects of dehydration on the surface morphology of articular cartilage. Journal of Orthopaedic Rheumatology. 6 (2-3), 75-80 (1993).
- Ateshian, G. A., Maas, S., Weiss, J. A. Solute transport across a contact interface in deformable porous media. Journal of Biomechanics. 45 (6), 1023-1027 (2012).
- Arbabi, V., Pouran, B., Weinans, H., Zadpoor, A. A. Multiphasic modeling of charged solute transport across articular cartilage: Application of multi-zone finite-bath model. Journal of Biomechanics. 49 (9), 1510-1517 (2016).
- Arbabi, V., Pouran, B., Zadpoor, A. A., Weinans, H. An experimental and finite element protocol to investigate the transport of neutral and charged solutes across articular cartilage. Journal of Visualized Experiments. 2017 (122), (2017).
- Sampson, S. L., Sylvia, M., Fields, A. J. Effects of dynamic loading on solute transport through the human cartilage endplate. Journal of Biomechanics. 83, 273-279 (2019).
- Bajpayee, A. G., Scheu, M., Grodzinsky, A. J., Porter, R. M. Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints. Journal of Orthopaedic Research : Official Publication of the Orthopaedic Research Society. 32 (8), 1044-1051 (2014).
- Bajpayee, A. G., et al. Sustained intra-cartilage delivery of low dose dexamethasone using a cationic carrier for treatment of post traumatic osteoarthritis. European Cells & Materials. 34, 341-364 (2017).
- Malda, J., et al. Of Mice, Men and Elephants: The Relation between Articular Cartilage Thickness and Body Mass. PLoS One. 8 (2), 57683 (2013).
- Frisbie, D. D., Cross, M. W., McIlwraith, C. W. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Veterinary and Comparative Orthopaedics and Traumatology. 19 (3), 142-146 (2006).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone